Anticoagulant Approved Guideline Community Clsi Org-PDF Free Download
Quick Guide: California’s Programs & Initiatives Advancing the Biopharmaceutical Industry STEM Workforce & Education: CLSI Bio-Community CLSI Bio-Link Equipment Depot CLSI’s Amgen Bay Area BioGENEius Challenge CLSI, The BioCollaborative CLSI, Biocom Institute – The Biotech Primer
Guidelines for the Management of Anticoagulant and Anti-Platelet Agent Associated Bleeding Complications in Adults Purpose: To be used as a common tool for all practitioners involved in the care of patients who present with bleeding problems related to use of anticoagulant and anti-platelet agents.
(A) Premature discontinuation of XARELTO increases the risk of thrombotic events Premature discontinuation of any oral anticoagulant, including XARELTO, increases the risk of thrombotic events. To reduce this risk, consider coverage with another anticoagulant if XARELTO is discontinued for a reason other than pathological bleeding or completion
Precision „ closeness of the agreement between the results of measurements of the same measurand repeatability reproducibility CLSI. User Protocol for Evaluation of Qualitative Test Performance; Approved Guideline - Second Edition. CLSI Document EP12-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2008.
May 2014 Approved Guideline 4th Edition M2-A12 Performance Standards for Antimicrobial Disk Susceptibility Tests Jan 2015 Approved Standard 12th Edition M39-A4 Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data Jan 2014 Approved Guideline 4th Edition
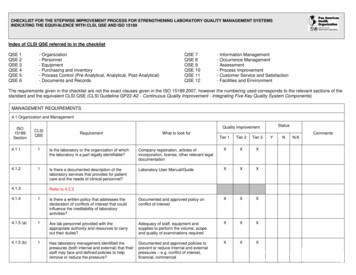
INDICATING THE EQUIVALENCE WITH CLSI, QSE AND ISO 15189 ISO 15189 Section CLSI QSE Requirement What to look for Quality Improvement Status Comments Tier 1 Tier 2 Tier 3 Y N N/A 4.2.4 1 Does the quality manual describe the quality management system and structure of the documentat
Virtual meetings of Working Groups and the AST Subcommittee are scheduled for August and September 2020. All are welcome, and free registration for web . ASTM International College of American Pathologists (CAP) European Committee on Antimicrobial Susceptibility Testing (EUCAST) Infectious Diseases Society of America (IDSA)
highly basic peptide that combines with HEPARIN as an ion pair lasts about 2 hours routinely used following cardiac surgery and other vascular procedures excess protamine also has an anticoagulant effect, since it can interact with platelets, fibrinogen and other plasma proteins
History of anticoagulant therapy 1910 1920 1930 1940 1950 1960 1970 1980 1990 2000 2010 Anticoagulant in spoiled sweet clover (K.P. Link) First clinical use of 4-hydroxycoumarin (O. Meyer et al) Warfar
the patient must contact the Anticoagulant team for advice. 10. The patient must not change the dose by themselves without instruction from the Anticoagulant team. This scheme supports self testing only not self management. 11. If the result is out of range the patient must con
Plasma Aqueous part of blood made noncoagulable by means of an anticoagulant such as EDTA, Heparin, or Citrate. Plasma can easily be obtained by placing whole blood into a tube containing an anticoagulant. When using a tube
KEY WORDS. Anticoagulant rodenticide, rat, mouse, mortalit ratey , LANIRAT-B. Of procedure aimes d at the eradication of commensal rodents (rat thos, mouse)e utilizin, onl chemicag y l . extensively used rodenticides may provoke the development of tolerance . bait formulas contained cor
Green tea and Clove. The aqueous extracts of Ginger, Garlic, Green tea and Clove were tested for in vitro prothrombin time (PT) test. The in vitro anticoagulant effects examined by using plasma, collected from blood samples of normal individuals by measuring PT. Ethylenediaminetetraacetic acid (EDTA) and saline in distilled water
tial diagnosis should include mild to moderate hereditary hemophilia, lupus anticoagulant, bleeding complications of anticoagulant treatments, trauma, NSAID abuse, and other acquired bleeding disorders (such as acquired von Willebrand disease and acquired platelet dysfunc
May 15, 2013 · Am J Health-Syst Pharm—Vol 70 May 15, 2013 Suppl 1 S3 Oral anticoagulant therapies: Balancing the risks Edith A. NutEscu Edith A. NutEscu, Pharm.D., FCCP, is Clinical Professor, College of Pharmacy, University of Illinois at Chicago, and Director, Antithrom-bosis Center, University of Illinois Hospital
Recent hip or knee replacement surgery: Blood clots may occur in people who are not physically mobile. People who have had a hip or knee replacement may need an anticoagulant to prevent blood clots until they are able to move around. OR Deep vein thrombosis (DVT): A blood clot in one of the deep veins in your body, usually in your leg. OR
Approved Data. Approved data means data approved by the FAA. The term “approved” is based on § 1.1, which states: “Approved, u\൮less used with reference to another person, means approved by the FAA or any person to whom the FAA has delegated its authority對 in the matter concerned, or approved under the provisions of a bilateral
Wood Ranch Architectural Guideline Addendum B Exterior Paint Palette/Technical Adjustments August 31, 2016 Approved & Adopted by BOD 8-31-16 8 Arch Guideline Addendum Exterior Color Palette 2016 31 APPROVED.docx 3 of 15 1.14.2a The Architectural Committee will make an investigation to verify the complaint is accurate.
This guideline replaces SIGN guideline 47 on preventing dental caries in children at high caries risk and SIGN guideline 83 on prevention and management of dental decay in the pre-school child. While the whole guideline has been newly developed, section 3 on predicting caries risk has been drawn from these previous guidelines. Section 3.4.1 has .
1.1 This guideline serves to set out the design parameters and inspection criteria applicable to facilities handling hormone products. This guideline’s primary focus is on the air-conditioning and ventilation systems of the facility. 1.2 This guideline is to be read in conjunction with other WHO good manufacturing practice
The following resource contains tables and figures from the 2018 Guideline for the Management of Blood Cholesterol. The resource is only an excerpt from the Guideline and the full publication should be reviewed for more tables and figures as well as important context. 2018 Guideline on the Management of Blood Cholesterol GUIDELINES MADE SIMPLE
The Michigan Parenting Time Guideline (Guideline) is produced by the Michigan Supreme Court, State Court Administrative Office. It provides information to help parents create a parenting time schedule in the best interests of their child. Parents might use the Guideline when trying to establish or modify a parenting time schedule or plan.
Technical Guideline for Indoor Repair, Storage and Cargo Handling for Vehicles Fuelled by Compressed Natural Gas and Liquefied Natural Gas Page 3 How to Use This Technical Guideline This document is intended to be a reference guideline related to facility design and operations issues at
of the Common Technical Document (ICH guideline M4). The guideline does not apply to contents of submissions for drug products during the clinical research stages of drug development. However, the principles in this guideline are important to consider during those stages as well. This guideline might also be appropriate for other types of products.
This guideline has been developed to assist in the design, maintenance, construction, and management of this infrastructure. 1.1 Purpose The purpose of this guideline is to detail minimum requirements to ensure that assets covered by the scope of this guideline are constructed and maintained to consistent standards and
AORN Guideline for Sterile Technique1 and the Guideline for Transmission-Based Precautions2 for additional infor-mation. The wearing of rings, bracelets, watches, nail polish, artificial nails, or other nail enhancements is outside the scope of this document; the reader should refer to the AORN Guideline for Hand Hygiene3 for additional .
This was the third guideline published under the Beyond the Pavement initiative. It accompanies Bridge Aesthetics, the Noise Wall Design Guideline and the Landscape Guideline and addresses the issue of the visual impact of shotcrete. The document has been updated to reflect lessons learned in cutting stabilisation over the past decade.
WHO handbook for guideline development . guidelines must follow current WHO guideline development standards as this handbook. In addition to being aware. outlined in of potential problems with regard to copyright and ownership, it is important to note that: hould be group; be guideline is ding fromFile Size: 850KB
Strategies for guideline development 105 The quality of the evidence 107 Grades of recommendation 108 Consumer involvement in guideline development 112 Guideline appraisal 113 The role of guidelines in practice 114 Practical tools for guideline development 115 Conclusion 116 8 Evidenc
2.1 Guideline 1: Maintain a Current Knowledge of Sustainability 6 2.2 Guideline 2: Integrate Sustainability into Professional Practice 6 2.3 Guideline 3: Collaborate with Peers and Experts from Concept to Completion 6 2.4 Guideline 4: Develop and Prepare
Evidence Based Clinical Guidelines 5 Methods used to update the guideline 6 Scope of the guideline 7 The Clinical Question 8 The Literature Search 8 The Appraisal Process 9 The Consensus Process 10 Good Practice Points (GPPs) 10 Drafting the updated guideline 11 Guideline Audit Tools 11
level reporting 56 Guideline 5: Assessment 86 Guideline 6: Liaison with the host school and/or the mainstream program 102 Guideline 7: Student care and welfare arrangements 112 Guideline 8: Record keeping 120 Intensive English Language Program (IELP) Guidelines 123 Part 4: Beyond the IELP: ESL support for international students 137
Adapted from Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2) Page 3. Malaysian Guideline for Good Clinical Practice, 4th Ed Malaysian Guideline for Good Clinical Practice 4 th Edition Publ
The guideline on Breast Cancer Screening and Diagnostics, published in 2000, was updated in 2007. In 2002, the first multidisciplinary National Breast Cancer Guideline was published, it was revised in 2004, 2005 and 2006. In 2008 both guidelines were combined to Breast Cancer Guideline, which 2012 revision is now effected. Objectives
This clinical practice guideline is based on the best available scientific evidence for the key questions as determined by the GDG. This means that our clinical practice guideline is not intended to replace the professional judgment of clinicians, but should help to inform clinical decision-making in particular clinical circumstances.
Financial Management (Sustainability) - 2 - The Guideline . This Guideline supersedes the. Financial Management (Sustainability) Guideline 2011, and is to be used in the calculation of the . relevant financial sustainability measures. detailed in Section 169(5) of the . Local Government Regulation 2012. and Section 160(5) of the
copies may be provided to patients and the clinicians who manage their care, if the ICSI Health . Care Guideline is incorporated into the medical group's clinical guideline program. All other copyright rights in this ICSI Health Care Guideline are reserved by the Institute for Clinical . Systems Improvement.
NEW ZEALAND SPEECH AND LANGUAGE THERAPY CLINICAL PRACTICE GUIDELINE INTRODUCTION Scope of the Guideline In 2009, the National SLT Health Leaders' Group identified the need for a New Zealand clinical guideline for speech-language therapists (SLTs) working with Videofluoroscopic Swallowing Study (VFSS), also known as
This guideline updates and replaces an earlier guideline published in 2003 from the AAFP and the American College of Physicians, which was reaffirmed by the AAFP in 2008.1 The topic was nominated to the Agency of Healthcare Research and Quality (AHRQ) for an updated evidence review in 2011. Changes in the methodology and scope of the guideline
1. Obtain a sample from the cervix according to the standard collection procedure (e.g., CLSI guideline GP15-A3).1 2. Holding the collection vial firmly down on a flat surface, insert the head(s) of the collection device(s) into the larger of the two openings in the BD SurePath Collection Vial using one of three methods: