Chapter 4 Atomic Structure Ponder Independent School-PDF Free Download
Physical & Chemical Properties Chemical & Physical Changes Matter Obj. 2.1.2 Atomic Structure Isotopes Matter Obj. 2.1.2 Rate Atomic Structure Obj. 2.1.4 Matter Obj. 2.1.2 Phase Change Test Matter Matter Atomic Structure Obj. 2.1.4 Atomic Structure Obj. 2.1.4 Atomic Structure Structure Atomic Structure Obj.
Part One: Heir of Ash Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 Chapter 24 Chapter 25 Chapter 26 Chapter 27 Chapter 28 Chapter 29 Chapter 30 .
TO KILL A MOCKINGBIRD. Contents Dedication Epigraph Part One Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Part Two Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18. Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 Chapter 24 Chapter 25 Chapter 26
DEDICATION PART ONE Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 PART TWO Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 .
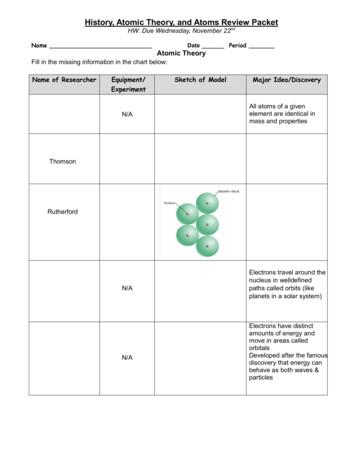
The Atomic Theory of Matter Dalton’s Atomic Theory Postulates 10 2. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of different elements are different. Different elements have different atomic properties such as atomic mass.
Atomic Structure Worksheet **Assume all are neutral atoms! Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number Protons Neutrons Electrons Mass number
About the husband’s secret. Dedication Epigraph Pandora Monday Chapter One Chapter Two Chapter Three Chapter Four Chapter Five Tuesday Chapter Six Chapter Seven. Chapter Eight Chapter Nine Chapter Ten Chapter Eleven Chapter Twelve Chapter Thirteen Chapter Fourteen Chapter Fifteen Chapter Sixteen Chapter Seventeen Chapter Eighteen
18.4 35 18.5 35 I Solutions to Applying the Concepts Questions II Answers to End-of-chapter Conceptual Questions Chapter 1 37 Chapter 2 38 Chapter 3 39 Chapter 4 40 Chapter 5 43 Chapter 6 45 Chapter 7 46 Chapter 8 47 Chapter 9 50 Chapter 10 52 Chapter 11 55 Chapter 12 56 Chapter 13 57 Chapter 14 61 Chapter 15 62 Chapter 16 63 Chapter 17 65 .
HUNTER. Special thanks to Kate Cary. Contents Cover Title Page Prologue Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter
Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 . Within was a room as familiar to her as her home back in Oparium. A large desk was situated i
5 Sec. 2 ATOMIC ENERGY ACT OF 1954 5 Section 3116(a)(1) of Public Law 104-134 (110 Stat. 1321-349) repealed chapters 22 through 26. CHAPTER 20. JOINT COMMITTEE ON ATOMIC ENERGY ABOLISHED; FUNCTIONS AND RESPONSIBILITIES REASSIGNED Sec. 301. Joint Committee on Atomic Energy Abolished. Sec. 302. Transfers of Certain Functions of the Joint Committee on Atomic Energy
IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 1.2.1 Slide 3 1.2 ATOMIC AND NUCLEAR STRUCTURE 1.2.1 Basic definitions for atomic structure There is no basic relation between the atomic mass number A and atomic number Z of a nucleus but the empirical re
The Hunger Games Book 2 Suzanne Collins Table of Contents PART 1 – THE SPARK Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8. Chapter 9 PART 2 – THE QUELL Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapt
History of the Atom- Chadwick Chadwick took Rutherford’s research further and found the neutron The problem was the atomic number was less than the atomic mass (average mass of the atom). For example, a helium atom has an atomic mass of 4, but an atomic number (or positive charge) of 2. Since electrons have almost no mass, it seemed
Lines 20-21: Atomic species declaration After the keyword ATOMIC_SPECIES, for each ntyp enter atomic symbol atomic weight pseudo-potential Lines 22-24: Atomic positions After the keyword ATOMIC_POSITIONS, for each nat enter atomic symbol x y z where x,y,z are given as fractional coordinates
shaped the atomic theory. Warm-Up Lesson Question Lesson Goals Atomic Theory Words to Know Fill in this table as you work through the lesson. You may also use the glossary to help you.? W 2K Describe the development of the . Explain how the atomic theory throughout history. How did the atomic theory
‘The Superheroes of the atomic model’ task covers the development of the atomic model in an active fact finding resource. This could be an initial lesson on the atomic model and the scientists that developed it into the current atomic
53.Gram atomic mass of oxygen 16g. 54.Gram atomic mass of sodium 23g. 55.Atomic mass is expressed in atomic mass unit (amu). 56.One atomic mass unit is defined as 1/12th part of the mass of one atom of carbon. 57.The relative molecular mass of an element o
Ancient atomic theory for the universe - Democritus & Epicurus (after 400 bC) Dalton atomic model (billiard ball model, 1803) Thomson atomic model . (Rutherford-Bohr model, 1913) Modern quantum mechanics (Heisenberg et al, 1927) TODAY. Timeline of Atomic Models 2 Democritus (460 b.C. - 370 b.C.): "Matter could not be divided into .
www.njctl.org Chemistry Atomic Structure Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does “n” stand for? 4. Using wavelengths emitte
Chapter 5 Atomic Structure and Processes 5.1 Elementary atomic structure . For multiple-electron atoms the electrons are said to reside in “shells”. These shells correspond to the principal quantum levels. The lowest energy states, corresponding to electrons bound most strongly to the nucleus, are the n 1 level, known as the K-shell. .
Chapter 9, end and Chapter 24. MO theory: Rules: 1. The number of MO's equals the # of Atomic orbitals 2. The overlap of two atomic orbitals gives two molecular orbitals, 1 bonding, one antibonding 3. Atomic orbitals combine with other atomic orbitals of similar energy. 4. Degree of overlap matters. More overlap means bonding
Page 1 of 3 1.4 w-1 Atomic Structure Worksheet.docx Atomic Structure Worksheet Fill in the blanks in the following table. Note: The mass may not match the mass on your periodic table. Assume all atoms are neutral Atomic symbol Atomic number Protons Neutrons Electrons Mass number C 8 12 24 31 40 40 89 30 35 42 98
Atomic Structure Early Atomic Theories Dalton’s Atomic Theory: Each element is composed of extremely small particles called atoms. All of the atoms of 1 element are identical to each other but are different from the atoms of another element. Atoms of one element cannot be changed into atoms of a different element by chemical
1. describe previous atomic theories and compare to our modern understanding of the atom (4.1) 2. distinguish among protons, electrons, and neutrons in terms of mass and charge. (4.2) 3. describe the structure of the atom. (4.2) 4. explain why isotopes differ and why atomic masses are not whole numbers. (4.3) 5. understand how atomic mass is .
Atomic Structure Timeline History of Atomic Theory. Essential Questions What does it mean when my science . perform controlled experiments like true scientists. Lavoisier 1777 French chemist, who is considered . This contradicted Dalton's Atomic Theory. This allowed a new model of the atom. J. J. Thomson (1903)
1. Introduction 2. Commentary 3. Questions to Ponder 4. Suggested Assignments 5. Additional Resources Introduction Each chapter begins with a brief intro-duction focusing on the central topics found in the chapter. Commentary A list of supporting scriptures to study and ponder often follows the main headings in the “Commentary” section.
Mary Barton A Tale of Manchester Life by Elizabeth Cleghorn Gaskell Styled byLimpidSoft. Contents PREFACE1 CHAPTER I6 CHAPTER II32 CHAPTER III51 CHAPTER IV77 CHAPTER V109 CHAPTER VI166 CHAPTER VII218 i. CHAPTER VIII243 CHAPTER IX291 CHAPTER X341 CHAPTER XI381 CHAPTER XII423 CHAPTER XIII450 CHAPTER XIV479 CHAPTER XV513 CHAPTER XVI551
Part Two: Heir of Fire Chapter 36 Chapter 37. Chapter 38 Chapter 39 Chapter 40 Chapter 41 Chapter 42 Chapter 43 Chapter 44 Chapter 45 Chapter 46 Chapter 47 Chapter 48 Chapter 49 Chapter 50 Chapter 51 . She had made a vow—a vow to free Eyllwe. So in between moments of despair and rage and grief, in between thoughts of Chaol and the Wyrdkeys and
atomic number 11 In the isotope symbol, the atomic number is written as a subscript (bottom left). Please note that in the periodic table, the atomic number is most often written above the symbol. In order to avoid making a mistake, please use the whole number (not the number with decimal
May 15, 2008 · CHAPTER THREE CHAPTER FOUR CHAPTER FIVE CHAPTER SIX CHAPTER SEVEN CHAPTER EIGHT CHAPTER NINE CHAPTER TEN CHAPTER ELEVEN . It is suggested that there is a one-word key to the answer among the four lofty qualities which are cited on every man's commission. . CHAPTER TWO. CHAPTER THREE.
the secret power by marie corelli author of "god's good man" "the master christian" "innocent," "the treasure of heaven," etc. chapter i chapter ii chapter iii chapter iv chapter v chapter vi chapter vii chapter viii chapter ix chapter x chapter xi chapter xii chapter xiii chapter xiv chapter xv
Chapter 1: Kinetic Particle Theory Chapter 2: Measurement and Experimental Techniques Chapter 3: Separation and Purification Chapter 4: Elements, Compound and Mixture Chapter 5: Atomic Structure Chapter 6: Chemical Bonding Chapter 7: Writing Chemical Equations Chapter 8: The Mole Chapter 9: Chemical Calculations Chapter 10: Acids and Bases
Scientists’ understanding about atomic structure has continued to evolve. Yet, we credit the Rutherford-Bohr Theory of Atomic Structure for providing us with a basis for understanding atomic structure. Atoms consist of proton
WEEK ONE/ UNIT ONE: Atomic structure (Sub-Topic: Sub-Atomic particles) Course Learning Outcome: (a) describe the structure of the atom in terms of the location of protons, neutrons and electrons in an atom (b) define terms associated with the numbers of protons , electrons and neutrons (c) describe the Bohr’s model of the atom .
topic 2: atomic structure – 7 Grade 12 C hemistry Topic 2: Atomic Structure The energB of the various parts of the electromagnetic (em) spectrum is directly related to the frequencB of the wave. If a wave has a high frequency, then it will contain a higher amount of
Topic: Atomic Structure Day 25 April 29 Google Classroom Topic: The Periodic Table Paper Pencil Topic: The Periodic Table Day 26-27 April 30-May 1 Google Classroom Topic: Test on the Atomic Structure & Periodic Table Paper Pencil Topic: Test on the Atomic Structure & Periodic Table Day 28-29 May 4-5 Google Classroom .
Trilogy C1: Atomic structure Collins revision guide: Atomic structure and the periodic table Knowledge Organiser Key points to learn 1. Atom Smallest part of an element that can exist Hydrogen atoms (4H) 2. Molecule Two or more atoms chemically bonded Hydrogen molecule (H 2) Water molecule (H 2 O) 3. Element Only one type or atom present. Can be
1.1.3 OCR Scheme of Work topic outlines 1. Historical development of atomic structure 2. Modern idea of atomic structure 3. Isotopes 4. Electronic structure of ions 5. Relative isotopic masses 6. Calculation of relative atomic masses 7. Relative formula/molecular masses Students should be able to: Describe how the model of the atom has
Atomic structure and the periodic table In this article, we hope to develop an understanding of atomic structure and the periodic table, from the beginning of secondary school/junior-high level, to first/second year undergraduate physics/chemistry, via one fictional conversation. We hope that people at any stage of that journey will find a .

















![Timeline of Atomic Models [3] - Unesp](/img/222/3aula-cme.jpg)




















