Chapter 7 Introduction To Atomic Spectroscopy-PDF Free Download
Part One: Heir of Ash Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 Chapter 24 Chapter 25 Chapter 26 Chapter 27 Chapter 28 Chapter 29 Chapter 30 .
TO KILL A MOCKINGBIRD. Contents Dedication Epigraph Part One Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Part Two Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18. Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 Chapter 24 Chapter 25 Chapter 26
DEDICATION PART ONE Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 PART TWO Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 .
Physical & Chemical Properties Chemical & Physical Changes Matter Obj. 2.1.2 Atomic Structure Isotopes Matter Obj. 2.1.2 Rate Atomic Structure Obj. 2.1.4 Matter Obj. 2.1.2 Phase Change Test Matter Matter Atomic Structure Obj. 2.1.4 Atomic Structure Obj. 2.1.4 Atomic Structure Structure Atomic Structure Obj.
About the husband’s secret. Dedication Epigraph Pandora Monday Chapter One Chapter Two Chapter Three Chapter Four Chapter Five Tuesday Chapter Six Chapter Seven. Chapter Eight Chapter Nine Chapter Ten Chapter Eleven Chapter Twelve Chapter Thirteen Chapter Fourteen Chapter Fifteen Chapter Sixteen Chapter Seventeen Chapter Eighteen
18.4 35 18.5 35 I Solutions to Applying the Concepts Questions II Answers to End-of-chapter Conceptual Questions Chapter 1 37 Chapter 2 38 Chapter 3 39 Chapter 4 40 Chapter 5 43 Chapter 6 45 Chapter 7 46 Chapter 8 47 Chapter 9 50 Chapter 10 52 Chapter 11 55 Chapter 12 56 Chapter 13 57 Chapter 14 61 Chapter 15 62 Chapter 16 63 Chapter 17 65 .
HUNTER. Special thanks to Kate Cary. Contents Cover Title Page Prologue Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter
Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 . Within was a room as familiar to her as her home back in Oparium. A large desk was situated i
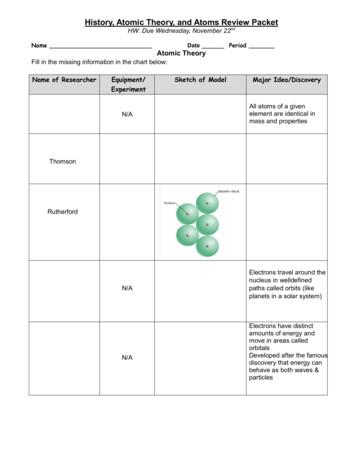
The Atomic Theory of Matter Dalton’s Atomic Theory Postulates 10 2. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of different elements are different. Different elements have different atomic properties such as atomic mass.
5 Sec. 2 ATOMIC ENERGY ACT OF 1954 5 Section 3116(a)(1) of Public Law 104-134 (110 Stat. 1321-349) repealed chapters 22 through 26. CHAPTER 20. JOINT COMMITTEE ON ATOMIC ENERGY ABOLISHED; FUNCTIONS AND RESPONSIBILITIES REASSIGNED Sec. 301. Joint Committee on Atomic Energy Abolished. Sec. 302. Transfers of Certain Functions of the Joint Committee on Atomic Energy
The Hunger Games Book 2 Suzanne Collins Table of Contents PART 1 – THE SPARK Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8. Chapter 9 PART 2 – THE QUELL Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapt
History of the Atom- Chadwick Chadwick took Rutherford’s research further and found the neutron The problem was the atomic number was less than the atomic mass (average mass of the atom). For example, a helium atom has an atomic mass of 4, but an atomic number (or positive charge) of 2. Since electrons have almost no mass, it seemed
Atomic Structure Worksheet **Assume all are neutral atoms! Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number Protons Neutrons Electrons Mass number
Lines 20-21: Atomic species declaration After the keyword ATOMIC_SPECIES, for each ntyp enter atomic symbol atomic weight pseudo-potential Lines 22-24: Atomic positions After the keyword ATOMIC_POSITIONS, for each nat enter atomic symbol x y z where x,y,z are given as fractional coordinates
shaped the atomic theory. Warm-Up Lesson Question Lesson Goals Atomic Theory Words to Know Fill in this table as you work through the lesson. You may also use the glossary to help you.? W 2K Describe the development of the . Explain how the atomic theory throughout history. How did the atomic theory
‘The Superheroes of the atomic model’ task covers the development of the atomic model in an active fact finding resource. This could be an initial lesson on the atomic model and the scientists that developed it into the current atomic
53.Gram atomic mass of oxygen 16g. 54.Gram atomic mass of sodium 23g. 55.Atomic mass is expressed in atomic mass unit (amu). 56.One atomic mass unit is defined as 1/12th part of the mass of one atom of carbon. 57.The relative molecular mass of an element o
Ancient atomic theory for the universe - Democritus & Epicurus (after 400 bC) Dalton atomic model (billiard ball model, 1803) Thomson atomic model . (Rutherford-Bohr model, 1913) Modern quantum mechanics (Heisenberg et al, 1927) TODAY. Timeline of Atomic Models 2 Democritus (460 b.C. - 370 b.C.): "Matter could not be divided into .
Chapter 9, end and Chapter 24. MO theory: Rules: 1. The number of MO's equals the # of Atomic orbitals 2. The overlap of two atomic orbitals gives two molecular orbitals, 1 bonding, one antibonding 3. Atomic orbitals combine with other atomic orbitals of similar energy. 4. Degree of overlap matters. More overlap means bonding
Mary Barton A Tale of Manchester Life by Elizabeth Cleghorn Gaskell Styled byLimpidSoft. Contents PREFACE1 CHAPTER I6 CHAPTER II32 CHAPTER III51 CHAPTER IV77 CHAPTER V109 CHAPTER VI166 CHAPTER VII218 i. CHAPTER VIII243 CHAPTER IX291 CHAPTER X341 CHAPTER XI381 CHAPTER XII423 CHAPTER XIII450 CHAPTER XIV479 CHAPTER XV513 CHAPTER XVI551
Part Two: Heir of Fire Chapter 36 Chapter 37. Chapter 38 Chapter 39 Chapter 40 Chapter 41 Chapter 42 Chapter 43 Chapter 44 Chapter 45 Chapter 46 Chapter 47 Chapter 48 Chapter 49 Chapter 50 Chapter 51 . She had made a vow—a vow to free Eyllwe. So in between moments of despair and rage and grief, in between thoughts of Chaol and the Wyrdkeys and
Class- VI-CBSE-Mathematics Knowing Our Numbers Practice more on Knowing Our Numbers Page - 4 www.embibe.com Total tickets sold ̅ ̅ ̅̅̅7̅̅,707̅̅̅̅̅ ̅ Therefore, 7,707 tickets were sold on all the four days. 2. Shekhar is a famous cricket player. He has so far scored 6980 runs in test matches.
May 15, 2008 · CHAPTER THREE CHAPTER FOUR CHAPTER FIVE CHAPTER SIX CHAPTER SEVEN CHAPTER EIGHT CHAPTER NINE CHAPTER TEN CHAPTER ELEVEN . It is suggested that there is a one-word key to the answer among the four lofty qualities which are cited on every man's commission. . CHAPTER TWO. CHAPTER THREE.
the secret power by marie corelli author of "god's good man" "the master christian" "innocent," "the treasure of heaven," etc. chapter i chapter ii chapter iii chapter iv chapter v chapter vi chapter vii chapter viii chapter ix chapter x chapter xi chapter xii chapter xiii chapter xiv chapter xv
work/products (Beading, Candles, Carving, Food Products, Soap, Weaving, etc.) ⃝I understand that if my work contains Indigenous visual representation that it is a reflection of the Indigenous culture of my native region. ⃝To the best of my knowledge, my work/products fall within Craft Council standards and expectations with respect to
IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 1.2.1 Slide 3 1.2 ATOMIC AND NUCLEAR STRUCTURE 1.2.1 Basic definitions for atomic structure There is no basic relation between the atomic mass number A and atomic number Z of a nucleus but the empirical re
atomic number 11 In the isotope symbol, the atomic number is written as a subscript (bottom left). Please note that in the periodic table, the atomic number is most often written above the symbol. In order to avoid making a mistake, please use the whole number (not the number with decimal
The Boron family. Element Atomic Number Electronic Configuration . (Ti) 81 1.2 Some Atomic and Physical Properties of Group 13 Elements Property Elements B Al Ga In Ti Atomic number 5 13 31 49 81 Atomic mass (g mol-1) 10.81 26.98 69.72 114.82 204 . Because B is so small that it cannot accommodation four
Atomic values The values of the 19 atomic types available in XML Schema E.g. xs:integer, xs:boolean, xs:date All the user defined derived atomic types E.g myNS:ShoeSize xs:untypedAtomic Atomic values carry their type together with the va
Book II Chapter I Chapter II Chapter III Chapter IV Chapter V Chapter VI Chapter VII Chapter VIII Chapter IX Chapter X Chapter XI Chapter XII Chapter XIII Chapter XIV Book III . The Storm and Stress period in German literature had been succeeded by the Romantic movement, but Goethe's classicism rendered him unsympathetic to it. Nevertheless .
CONTENTS Introduction Chapter 1: Chapter 2: Chapter 3: Chapter 4: Chapter 5: Chapter 6: Chapter 7: Chapter 8: Chapter 9: Chapter 10: Chapter 11: Chapter 12: Chapter .
The total attenuation cross-section and effective atomic number are basic quantities required in determining the penetration of X-ray and gamma rays in matter [1]. The knowledge of mass attenuation coefficients, atomic and electronic cross sections and effective atomic number is useful for understanding * Corresponding author. Tel.: 6-683-287 .
b. Terminology and notation of nuclear physics.—There are three parameters used to characterize the nucleus: atomic number (Z), neutron number (N), and atomic mass number (A). The atomic number Z is the number of p
Atomic mass is the mass of an atom in atomic mass units (amu) Micro World atoms & molecules Macro World grams microworld macroworld Atomic mass ! mass of an atom in atomic mass units (u) u u u L. A. Anchordoqui (CU
ATOMIC PHYSICS TESTS OF THE STANDARD MODEL, C s NUCLEUS ATOMIC PHYSICS TESTS OF THE STANDARD MODEL, C s NUCLEUS Standard Model QW 73.16(3) 1999 analysis of Cs experiment showed 2.5 σ deviation from the Standard Model Most current result: Atomic physics [1] QW 73.16(29) exp (20) th
PROBLEM SOLUTIONS Fundamental Concepts Electrons in Atoms 2.1 Cite the difference between atomic mass and atomic weight. Solution Atomic mass is the mass of an individual atom, whereas atomic weight is the average (weighted) of the
atomic mass or atomic number the exercise is focused on alpha and beta emissions. Key Alpha particle: Beta particle: Gamma ray: In the example, Rn is the atomic symbol for the element Radon. The number 222 indicates the atomic mass of the element (or isotope). The number 86 respresents the element’s
Page 1 of 3 1.4 w-1 Atomic Structure Worksheet.docx Atomic Structure Worksheet Fill in the blanks in the following table. Note: The mass may not match the mass on your periodic table. Assume all atoms are neutral Atomic symbol Atomic number Protons Neutrons Electrons Mass number C 8 12 24 31 40 40 89 30 35 42 98
AAnalyst 200/400 Atomic Absorption Spectrometers 11 PinAAcle 900 Atomic Absorption Spectrometers 11 Optima 8x00 ICP-OES Spectrometers 11 NexION 300 ICP-MS Spectrometers 12 Importance of Atomic Spectroscopy To Specific Markets 13 Atomic Spectroscopy Detection Limits
Atomic Structure Early Atomic Theories Dalton’s Atomic Theory: Each element is composed of extremely small particles called atoms. All of the atoms of 1 element are identical to each other but are different from the atoms of another element. Atoms of one element cannot be changed into atoms of a different element by chemical
















![Timeline of Atomic Models [3] - Unesp](/img/222/3aula-cme.jpg)





















