Laboratory Manual For Acid Base Titration-PDF Free Download
Acid 1 to Base 1 - acid that gives up proton becomes a base Base 2 to Acid 1 - base that accepts proton becomes an acid Equilibrium lies more to left so H 3O is stronger acid than acetic acid. Water can act as acid or base. Acid 1 Base 2 Acid 2 Base 1 H 2O NH 3 NH 4 OH-
Bruksanvisning för bilstereo . Bruksanvisning for bilstereo . Instrukcja obsługi samochodowego odtwarzacza stereo . Operating Instructions for Car Stereo . 610-104 . SV . Bruksanvisning i original
Drill: Identify the B-L acid and base in each of the following. Circle any amphoteric species acid acid base base Note: In both examples, water behaved as an acid or a base. A species that can act as an acid or a base is called amphoteric. Bronsted-Lowry (B-L) Theory – Cont. acid base 1) HNO 3
10 tips och tricks för att lyckas med ert sap-projekt 20 SAPSANYTT 2/2015 De flesta projektledare känner säkert till Cobb’s paradox. Martin Cobb verkade som CIO för sekretariatet för Treasury Board of Canada 1995 då han ställde frågan
service i Norge och Finland drivs inom ramen för ett enskilt företag (NRK. 1 och Yleisradio), fin ns det i Sverige tre: Ett för tv (Sveriges Television , SVT ), ett för radio (Sveriges Radio , SR ) och ett för utbildnings program (Sveriges Utbildningsradio, UR, vilket till följd av sin begränsade storlek inte återfinns bland de 25 största
Hotell För hotell anges de tre klasserna A/B, C och D. Det betyder att den "normala" standarden C är acceptabel men att motiven för en högre standard är starka. Ljudklass C motsvarar de tidigare normkraven för hotell, ljudklass A/B motsvarar kraven för moderna hotell med hög standard och ljudklass D kan användas vid
LÄS NOGGRANT FÖLJANDE VILLKOR FÖR APPLE DEVELOPER PROGRAM LICENCE . Apple Developer Program License Agreement Syfte Du vill använda Apple-mjukvara (enligt definitionen nedan) för att utveckla en eller flera Applikationer (enligt definitionen nedan) för Apple-märkta produkter. . Applikationer som utvecklas för iOS-produkter, Apple .
In this experiment an acid-base titration will be used to determine the molar concentration of a sodium hydroxide (NaOH) solution. Acid-base titrations are also called neutralization titrations because the acid reacts with the base to produce salt and water. During an acid-base titration, there is a point when the number of moles of acid (H ions)
Acid-bases occur as conjugate acid-base pairs. CH 3 COOH and CH 3 COO-are a pair. H 2 O and H 3 O are a pair. The conjugate base of an acid is the base that is formed when the acid has donated a hydrogen ion. The conjugate acid of a base is the acid that forms when base accepts a hydrogen ion. Example 2 Which are Br Ø nsted-Lowry acids and .
As in the weak acid-strong base titration, there are three major differences between this curve (in blue) and a strong base-strong acid one (in black): (Note that the strong base-strong acid titration curve is identical to the strong acid-strong base titration, but flipped vertically.) 1- The weak-acid solution has a lower initial pH.
for pig slurry, and lactic acid sulfuric acid acetic acid citric acid for dairy slurry. In contrast, when the target pH was 3.5, the additive equivalent mass increased in the following order, for both slurries: sulfuric acid lactic acid citric acid acetic acid; acidification of pig slurry with all additives significantly (p 0.05)
Acid-Base Accounting: What is it? Acid-Base Accounting (ABA) is the balance between the acid-production and acid-consumption properties of a mine-waste material. Minerals in waste material (mostly sulfides; mostly pyrite) react with water and oxygen to produce sulfuric acid. This acid is
be hard or soft and also be either weak or strong. In a competition reaction between two bases for the same acid, one must consider both the relative strength of the bases, and the hard/soft nature of each base and the acid. ZnO 2LiC4 H9 Zn C 4 H9 Li2 O borderline acid hard base hard acid soft base borderline acid soft b
An acid/base neutralization reaction will yield salt and water. In an acid-base titration, the neutralization reaction between the acid and base can be measured with either a color indicator or a pH meter. . Four lab periods assigned for this experiment. In part I you will prepare an acid (HCl) solution and a base .
2/6/2004 OFB Chapter 8 4 Acid-Base Equilibria Brønsted-Lowry Acids and Bases A Brønsted-Lowry acid is a substance that can donate a hydrogen ion. A Brønsted-Lowry base is a substance that can accept a hydrogen ion. In the Brønsted-Lowry Acid and Base concept, acids and bases occur as conjugate acid-base pairs.
The third common factor has a larger load on the two variables of malic acid and hue. Malic acid is known to be a natural acid that balances the sweetness of wine. Malic acid is commonly used in the production of wines for lactic acid fermentation (MLF), in which lactic acid bacteria convert the more acidic malic acid into less acidic lactic acid.
Titration of amino acid: When an amino acid is dissolved in water it exists predominantly in the . isoelectric form. Amino acid is an . amphoteric. compound It act as either an acid or a base: Upon titration with acid it acts as a BASE (accept a proton). Upon titration with base it acts as an ACID (donate a proton)
different theories. An acid yields hydrogen ions 2. in aqueous solution. An Arrhenius base yields in aqueous 3. solution. A Brønsted-Lowry acid is a donor. A Brønsted-4. Lowry base is a proton . In the Lewis theory, an acid is an 5. acceptor. A Lewis base is an electron-pair . 6. An acid with one ionizable hydrogen atom is called a 7
There are 3 regions, or 3 different types of calculations, in a strong acid/strong base titration curve: A. Before the equivalence point - in this example not all H is reacted. . Section 3 - Weak Base/Strong Acid titration 1. Before adding acid: A weak base problem. 2. After adding acid but before equivalence point: A
hydrobromic acid, used industrially to prepare bromide compounds TRY IT FIRST SOLUTION a. 1. acid, phosphoric acid 2. base, sodium hydroxide b. 1.Mg(OH) 2 2. HBr STUDY CHECK 14.1 a. Identify as an acid or a base and give the name for H 2CO 3. b. Write the formula for iron(III) hydroxide. ANSWER a. acid, carbonic acid b. Fe(OH) 3 Naming Bases
Aunty Acid Breaks the Internet, 19 Aunty Acid Laugh ’til You Leak!, 142 Aunty Acid’s Getting Older, 19, 142 Aunty Acid’s Guide to Life, 142 Aunty Acid’s Guide to Love, 142 Aunty Acid’s Office Manual, 142 Aunty Acid With Age Comes Wisdom, 142 Autograph Book of L.A., The, 151 A
Lewis Base Lewis Acid Tetrafluoroborate ion Electron deficient compounds, which can behave a electron pair acceptors are Lewis acids. A species that donates an electron pair is a Lewis base. . Br Br Conjugate Acid-Base Pairs Base Acid Conjugate acid Conjugate base 1. Note that the H-Br b
article describes how acid rain is formed, what the difference is between acid rain and acid deposition, and the effects of acid rain on nature and humans. The article also provides access to a nationwide network of acid rain monitoring stations which are updated weekly. The article gives a good overview of the basics of acid deposition.
och krav. Maskinerna skriver ut upp till fyra tum breda etiketter med direkt termoteknik och termotransferteknik och är lämpliga för en lång rad användningsområden på vertikala marknader. TD-seriens professionella etikettskrivare för . skrivbordet. Brothers nya avancerade 4-tums etikettskrivare för skrivbordet är effektiva och enkla att
Den kanadensiska språkvetaren Jim Cummins har visat i sin forskning från år 1979 att det kan ta 1 till 3 år för att lära sig ett vardagsspråk och mellan 5 till 7 år för att behärska ett akademiskt språk.4 Han införde två begrepp för att beskriva elevernas språkliga kompetens: BI
**Godkänd av MAN för upp till 120 000 km och Mercedes Benz, Volvo och Renault för upp till 100 000 km i enlighet med deras specifikationer. Faktiskt oljebyte beror på motortyp, körförhållanden, servicehistorik, OBD och bränslekvalitet. Se alltid tillverkarens instruktionsbok. Art.Nr. 159CAC Art.Nr. 159CAA Art.Nr. 159CAB Art.Nr. 217B1B
produktionen sker på ett reproducerbart sätt. Alla geler som produceras testas därför för att kontrollera att de upprätthåller den kvalité som krävs för produktion av läkemedel. De biologiska läkemedlen kan sorteras på olika egenskaper och för geler som separerar med
An Asahi Kasei Group Company Inledning Den här manualen innehåller handhavandeinstruktioner för webbportalen Senseair Dashboard med dess användare som tänkta läsare. Inledningsvis beskrivs några begrepp som lägger grunden för behörigheter i systemet. Därefter följer steg för steg instruktioner av alla funktioner i systemet.
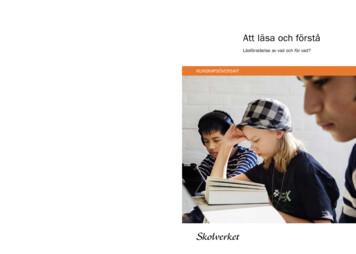
Att läsa och förstå Läsförståelse av vad och för vad? Att läsa och förstå. Skolverket. KUNSKAPSÖVERSIKT KUNSKAPSÖVERSIKT. Forskning visar att läsförståelsen påverkar möjlig heterna att tillägna sig kunskaper i alla skolämnen. Men vad kan skolan göra för att stödja elevers läs förståelse genom hela grundskolan?
This presentation and SAP's strategy and possible future developments are subject to change and may be changed by SAP at any time for any reason without notice. This document is 7 provided without a warranty of any kind, either express or implied, including but not limited to, the implied warranties of merchantability, fitness for a .
Acid-Base Titrations Purpose: The purpose of this lab is to determine the equivalent mass and pK a of the unknown acid. In addition, the NaOH will be used to verify the equivalent mass of unknown acid B. Lastly, the lab will allowed the pK a of the unknown acid to be determined from the graph of pH and the volume of strong base added.
For an acid-base titration, the equivalence point occurs when moles of acid equal moles of base: [H 3O ] [OH-]. Furthermore, the equivalence point will reveal whether the solution consists of a strong or weak acid. For an acid, HA, in solution, the equilibrium constant K a for the process can be determined: HA (aq) H 2O (l) H
II. Weak acid- strong base titration In this part of the experiment you will do weak acid strong base titration. 0.05M, 20 mL CH 3 COOH is titrated with 0.05M NaOH. perform same procedure mentioned above. III. Mixture of weak acid, strong acid and strong base titration 0.02M, 10 mL HCl and 0.02M, 10 ml acetic acid mixture is titrated with 0.05 .
Instead of sulfuric acid, this lab involves two different acids: citric acid and ascorbic acid (both are acids, thus each reacts with NaOH). You can determine the TOTAL amount of acid (total moles of H moles of H from citric acid moles of H from ascorbic acid) present in a juice sample by titration with NaOH, a strong base. Equation 1
CO 2-CH3NH3 CH3NH2 H2 O HO-CH3 CH2OH 3 O-HC CH HC C-H2 H-NH3 NH2-CH 2 CH 2 CH 2 CH-CH3CH3 CH3CH2-Acid Formula pK a Conjugate Base Ethane Ammonia Ethanol Water Bicarbonate ion Phenol Ammonium ion Carbonic acid Acetic acid 35 25 Benzoic acid Phosphoric acid Sulfuric acid Hydrogen chloride Hydrogen bromide Hydrogen iodide 51 38 10.33 15.7 15.9 .
Titration of strong acid with strong base 0 2 4 6 8 10 12 14 0 10 20 30 40 50 ml base added pH eq. pt. pH 7.00 F a 0.01 M V a 40 ml F b 0.02 M At equiv. point: V b (V a*M a)/M b 20 ml Strong acid / strong base titration remaining strong acid determines pH excess strong base determines pOH and, hence, the pH Formal amount of acid in initial solution
Acid-Base Theories 25. Classify each as an Arrhenius acid or an Arrhenius base. a. Ca(OH) 2 c. KOH e. HBr b. HNO 3 d. C 2 H 5 COOH f. H 2 SO 4 26. Identify the acid, base, conjugate acid and conjugate base in the following equations. a. HNO 3 H 2 O H 3 O NO 3-b. CH 3 COOH H 2 O H 3 O CH 3
Acid-Base Equilibria – Acids - sharp, sour taste; Bases - soapy, bitter taste – Neutralization (proton transfer) reactions acid base salt water (or other products) – Proton (H ) – strongly hydrated in water H(H 2O)n – Hydronium ion – H3O 18.1 Acid-Base Definitions
- From Arrhenius to Lewis, the definitions get broader as you go along. In other woeds, the later definitions include MORE SUBSTANCES under the acid/base umbrella. If something is an Arrhenius acid, it is also an acid in the Bronsted or Lewis picture. If something is an Arrhenius base, it is also a base in the Bronsted or Lewis picture.File Size: 400KB
reaction in this problem, is (1)—a Lewis acid–base reaction. (a) In general, if a reaction in one direction is a Brønsted acid–base reaction, it is also a Brønsted acid–base reaction in the reverse direction. Therefore, Steps 1 and 3 are (5)—Brønsted acid–base




















![INDEX [gibbssmithcovers ]](/img/116/gs-s2020-catalog-back.jpg)