Formal Analysis Of Isothermal Martensite Spread
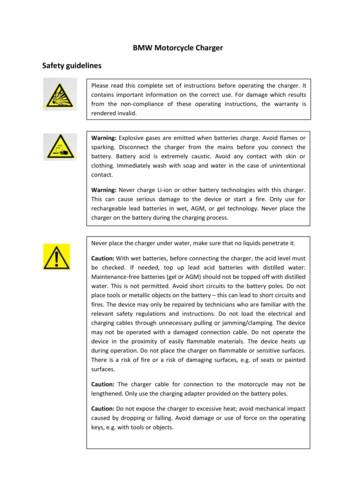
Materials Research, Vol. 11, No. 1, 103-108, 2008 2008Formal Analysis of Isothermal Martensite SpreadPaulo Rangel Riosa*, José Roberto Costa Guimarãesa,bEscola de Engenharia Industrial Metalúrgica de Volta Redonda,Universidade Federal Fluminense – UFF,Av. dos Trabalhadores, 420 27255-125 Volta Redonda - RJ, BrazilbMal. Moura 338h/22c 05641-000 São Paulo - SP, BrazilaReceived: December 4, 2007; Revised: March 19, 2008A formal kinetic approach was applied to the spread of isothermal martensite over the neighboring austenitegrains in a Fe-23.2 wt. (%) Ni-2.8 wt. (%) Mn alloy. The number of grains in a spread event changed with parentaustenite grain size. However, isothermal martensite spread formed from fine-grained parent austenite andathermal martensite from a Fe-31 wt. (%) Ni-0.02 wt. (%) C alloy studied in a previous work followed the samemicrostructural path. The number of grains per spread- event found in the present study was shown to be consistentwith the number of neighbors of the grain originating the spread event by means of a simple geometrical modelof the parent austenite grain network. The study of the kinetics of isothermal martensite spread showed that thenucleation rate of the spread-event in isothermal martensite remained constant during the transformation. Thisresult parallels the constant nucleation rate of the spread-event also found using the same methodology in athermalmartensite formed in a Fe-31 wt. (%) Ni-0.02 wt. (%) C alloy studied in a previous work.Keywords: martensitic phase transformation, kinetics, microstructure, analytical methods, grain-size effects1. IntroductionMartensite is a displacive phase transformation with significanttransformation strains which prompts microstructural heterogeneity,influences plate’s shape, and can lead to diversity in kinetics. Usually,the martensite reaction does not start simultaneously in all austenitegrains. In bulk polycrystals, the first nucleation event in a single grainmay induce transformation in neighboring grains, resulting in a cluster ofpartially transformed grains. This cluster of partially transformed grainsis normally designated as a ‘spread event’. The collection of these singlespread events can be defined as the ‘spread’ or ‘martensite spread’. Themartensite reaction then proceeds by nucleation of additional martensiteunits within the partially transformed grains, in what is normally calledthe ‘fillin-in’. Martensite nucleation events may take place in a numberof grains leading to a certain number of clusters or spreads. In athermalmartensite this nucleation takes place very fast at a certain temperature,as a consequence spread and fill-in are completed almost instantaneously.By contrast, in isothermal martensite, nucleation of new martensite platesin untransformed grains and the consequent spread event takes place asa function of reaction time. As a consequence, in isothermal martensite,martensite spread can be followed as function of time.The microstructure of the martensitic spread is described by thevolume fraction of material in grains partially transformed, Vgv (notthe martensite volume fraction) and by the area of interface betweena cluster of partially transformed grains and the untransformed parent matrix, per unit volume of material, Sgv. All symbols used in thispaper are defined in Table 1 for convenience. The martensite burstis the classical example of autocatalytic spread of martensite over alarge number of neighboring austenite grains in a single event. Anoptical photomicrograph of a Fe-31 wt. (%) Ni-0.01 wt. (%) C alloypartially transformed by cooling to its burst temperature, MB 220 K,is shown in Figure 1. Clusters of partially transformed grains in amatrix of untransformed grains are conspicuous.The martensitic spread in materials that isothermally transform toplate martensite at sub-zero temperatures is the topic of this communication. The current analysis is based on plate martensite formation in alloys*e-mail: prrios@metal.eeimvr.uff.brof high Ni and high Ni plus Mn contents with sub-zero transformationtemperatures. The present analysis does not apply to lath martensiteformation in Fe-C alloys or steels with high Ms temperatures.In a recent paper1, the present authors have shown that themethodology of formal kinetics was useful to deal with spread inathermal martensite. The basis of formal kinetic modeling is the earlywork of Johnson-Mehl2, Avrami3 and Kolmogorov4 (JMAK ), whichused only a single microstructural descriptor, VV. JMAK’s work wassubsequently extended by DeHoff and Gokhale’s microstructural pathmethod (MPM)5-8, who proposed the use of an additional microstructural descriptor, SV, and the associated concept of microstructuralpath. Vandermeer and coworkers9 extended DeHoff’s microstructuralpath concept and crystallized it in an all round theoretical treatmentcovering variable nucleation and growth rates as well as non-sphericalregions: the microstructural path method (MPM).The microstructural path method was originally developed to dealwith recrystallization and later applied also to diffusional transformations and grain growth but it is in fact quite general and can in principlebe used to model any heterogeneous transformation.In this work, the evolution of spread during isothermal martensite transformation is modeled with the help of the formal kineticsmethodology. The theoretical results will be checked with experimental data obtained from Fe-23.2 wt. (%) Ni-2.8 wt. (%) Mn10,11isothermally transformed.Martensite reaction is nucleation-controlled. Preferred nucleationsites are postulated to exist dormant in the material and propagate thepioneer nucleation event in untransformed grains under the availablechemical driving force. Their potency, however, is not uniformlydistributed. The reaction inherits this heterogeneity and it is firstlyobserved only in a few austenite grains12. Nonetheless, the pioneernucleation in a grain has influence over a certain spread volume, vsp,comprising a number γ of austenite grains.vsp q g(1)
104Materials ResearchRios & GuimarãesTable 1. List of symbols used in this paper and their definitions.SymbolDefinitionMsMartensite start temperature;VvMartensite volume fraction;VgvVolume fraction of material in grains partially transformed grains, that is, volume fraction of all grains in allclusters of martensite spread;SgvArea of interface per unit volume of material between the clusters of partially transformed grains and theuntransformed parent matrix, that is, area of interface per unit volume of martensite spread;Vv,ext, Vgv,ext , Sgv,extThe subscript ext added to these quantities mean that they are extended quantities, that is, that they are calculatedignoring impingement among growing regions.vspVolume of a single cluster of austenite grains partially transformed to martensite comprising a number γ ofaustenite grains, that is, volume of a single spread event;sspSurface area between a single cluster of austenite grains partially transformed to martensite and the untransformedmatrix grains, that is, surface area of a single spread event;kShape factor related to a single spread event or cluster of partially transformed austenite grains;γNumber of grains in a cluster of partially transformed austenite grains that constitute a spread event;Coefficient obtained from microstructural path, a 2g–1/3 ;aNNumber of austenite grains per unit volume in the parent austenite matrix;qAverage austenitic grain volume, NVm 1/q;mvdGrain diameter, d (6q/π)1/3SmvGrain boundary area per unit volume of parent austenite matrix grains;λMean intercept length of parent austenite matrix intercept, Smv 2/λ;nvTotal number per unit volume of active nuclei at a certain temperature;nInitial density of pre-existent, dormant sites at a certain temperature;nValue of nv at Ms temperature;nNumber of pre-existent nucleation sites at t Ms-T, where T is the transformation temperature;i,TvMsv TvIvNucleation rate,;t0Incubation time;mVolume fraction of martensite in a single grain resulting from the first martensite plate to form;α, β, RParameters of linear regression:,α is the intercept and β is the slope of the straightline, R is the correlation coefficient;where q is the average austenitic grain volume, q 1/ Nmv where Nmvis the number of austenite grains per unit volume. The transformationcontinues by subsequent “spread events” that spread the reaction tothe untransformed grains.2. Experimental Data100 MmFigure 1. Optical micrograph of a Fe-31 wt. (%) Ni-0.01 wt. (%) C alloytransformed at Ms (220 K). The martensitic ‘spread’ can be clearly seen againstthe background of austenite grains.The data used here were obtained by quantitative metallographictechniques to determine volume fraction and interface area per unitof volume from planar sections. The highly pure FeNiC and FeNiMnalloys were reacted under strict control as described in the referencedpapers. The authors10,11 have described and discussed their experimental conditions at length and that will not be repeated here for brevity.However, for the present work, the data were compiled by scanningand digitizing their graphs with relevant metallographic quantities.Non-conspicuous data points were dismissed. These data were consolidated by reiteration to average out small variations. In the following, FeNiC refers to Fe-31 wt. (%) Ni-0.02 wt. (%) C and FeNiMnrefers to Fe-28 wt. (%) Ni-3 wt. (%) Mn. Svm was obtained from themean intercept length with the well-known13 stereological relation-
Vol. 11, No. 1, 2008105Formal Analysis of Isothermal Martensite Spreadship Svm 2/λ. The values of q were calculated from λ assuming thatparent austenite grains can be approximated by tetrakaihedra13 (SeeFigure 4 and discussion in Section 5.): q 2.34 λ3 ; d, the diameterof a sphere with a volume equal to the grain volume, can be found byd (6 q/p)1/3. The values of λ, Svm,q and d are listed in Table 2 for theFeNiMn alloy. In his original work, Ghosh10,11 gives the mean interceptlength, λ, for each alloy.3. Microstructural Path AnalysisThe MPM describes the transformation in extended space. Theextended quantities are transformed into real measurable quantitiesby means of the fundamental relationshipsVgv –exp(–Vgv,ext)(2)(3)where Sgv,ext and Vgv,ext are extended quantities.Rios and Guimarães1 have derived an expression for the microstructural path of the athermal martensite spread. They assumed arelationship between the volume and surface area of the individualspread, vsp and ssp, respectively. This relationship is9Ssp kv2/s 3p(4)where k is a shape factor. For a tetrakaihedron shape one obtainsk 5.31 whereas for a sphere: k 4.8. In extended space, nv isdefined as the total number per unit volume of nuclei which havebecome active during cooling to the temperature of interest. Usingnv, the extended quantities are given by Vgv,ext vsp nv and Sgv,ext ssp nv.Inserting these into Equation 4 results in13(5)Equation 5 is the microstructural path in extended space. It canbe converted to the microstructural path in real space with the helpof Equations 2 and 3Another possibility would be to assume a spherical shape andin this case(8)where the subscript ‘s’ in γs is meant to emphasize that it was obtainedfrom the assumption of a spherical shape. A relationship between γsand γ can be easily found: gs 0.73 g. We will use γ throughout thispaper for consistency with previous work but for comparison purposesit is also interesting to have γs.It is worthy of note that the derivation of Equation 6 did not makeany specific kinetic assumptions concerning athermal martensite butonly assumptions regarding the spread volume, Equation 1. Therefore, Equation 6 should also be applicable for isothermal martensiteand possibly to mechanically induced martensite as well. Figure 2confirms that the experimental data can be well described by themicrostructural path, Equation 6.Both isothermal sets of data plotted after Equation 6 can bedescribed by a best-fit straight line passing through the origin witha slope a equal to 0.49 for d 0.031 mm and a equal to 0.75 ford 0.079 mm, with a high correlation coefficient, R 0.98 and 0.99,respectively. The values of γ, calculated using Equation 7, were equalto 68 (gS 50) for the fine-grained austenite and 19 (gS 14) for thecoarse-grained austenite and are listed in Table 2. Previous analysisof athermal martensite in Fe-31 wt. (%) Ni-0.02%C showed that theparameter a was also equal to 0.49 and consequently γ was also equalto 68 and independent of grain size. By contrast, in the isothermaltransformation analyzed here the values of γ depended on the grainsize. The reason for this is discussed in section 5 below. For thefine-grained matrix, d 0.031 mm, the values of a 0.49 and γ 68obtained by MPM were identical to those obtained for the athermalmartensite in Fe-31 wt. (%) Ni-0.02%C1. The data corresponding tothe athermal martensite of Fe-31 wt. (%) Ni-0.02%C1,14,15 are alsoincluded in Figure 2. Notice that the theoretical MPM line is commonto both fine-grained isothermal and athermal martensite data sincetheir values of a were identical as already mentioned. Therefore,it is evident from Figure 2 that the fine-grained isothermal and theathermal martensite spreads follow the same microstructural path(6)Vvg0.00a 2 g–1/3(7)Table 2. Fe-28 wt. (%) Ni-3 wt. (%) Mn for Ghosh10,11 - isothermallytransformed at 153 K - γ, γs, Ιv and t0,lower obtained from the analysis ofexperimental data.λmm0.0190.048dSmvmm–1 mm105.0 0.03141.7 0.079qmm31.6 10–52.6 4171.5(Svg/Svm)/(1 Vvg)where Smv , the total area of austenite grain boundaries per unit of volumeof material. The constantcan be simplified assumingthat the parent austenite grains can be approximated by tetrakaihedra13S mv 2.6 (Nmv ) 1/3 and remembering that Vsp g(Nmv )–1.The parameter a in Equation 6 depends only on γ, which on itsturn depend on the parameter k. The parameter k is a function of theshape of the spread volume. One possible assumption, adopted inour earlier paper was that the spread volume had a tetrakaidecahedralshape, thus leading to1.00.390.780.860.92IsotermalFe-28 wt. (%) Ni-3 wt. (%) Mnd 0.031 mma 0.49 R 0.98d 0.079 mma 0.75 R 0.990.50.00.00.63AthermalFe-31 wt. (%) Ni-0.02 wt. (%) Cd 0.080 mmd 0.043 mm0.51.01.52.02.5ln (1/(1 Vvg))Figure 2. Microstructural path of isothermal martensite spread. The theoreticalresult, Equation 6, is compared with experimental data from a Fe-23.4 wt. (%)Ni-2.8 wt. (%) Mn alloy obtained by Ghosh10,11. The correlation coefficient,R, is also shown. The fine-grained isothermal martensite and the athermalmartensite follow the same microstructural path.
106Materials ResearchRios & Guimarãesregardless of their significant differences in kinetics particularlywith regard to the number of martensite units per grain in a spreadevent10,16. This result is remarkable and indicates a basic formalsimilarity between the special aspects of martensite spreads in suchapparently different reactions.4. Formal Analysis of Spread KineticsWhereas pioneer isothermal martensite nucleation takes place atthe most potent pre-existent nucleation sites, autocatalytic nucleationpromoted by the martensite plate in its vicinity has a clear effect onfilling-in with martensite units the partially transformed grains andalso on poking the reaction into a next grain. In extended space, thenumber per unit volume of pre-existent nucleation sites which havebecome active as a function of time at temperature of interest,nv, isgiven bydnv (t) Iv (t) dt(9)(17)In these plots, the values of γ used, were obtained from MPM analysis, see Table 2.From these straight lines, β can be immediately recognized to beequal to the nucleation rate, Iv . Unfortunately, one cannot extract thevalues of ni,Tand t0 from this regression. This problem can be bettervunderstood rewriting the right hand side of Equation 16, using, Iv band comparing it with Equation 11– Ivt0a ni,Tv(18)It can be seen from Equation 18 that there are two unknowns:and t0 and only one experimental parameter, α. Under these cirni,Tvcumstances, one may obtain only a lower bound for the incubationtime, t0,lower , by setting the volume fraction of partially transformedgrains equal to zeroor(19)(10)where Ivis rate of that process. It is important to stress that no assumptions are made here concerning the specific time dependenceof either nvor Iv.However, one must consider that there is an incubation time, t0, atwhich a pioneer nucleation event takes place at one of the pre-existentnucleation sites. The initial density of these pre-existent, dormant. Thereafter, follows Eqution 10. Therefore Equation 10sites iscan be rewritten as(11)Here, γ is considered to remain constant independent of transformation temperature, time or fraction transformed but may depend ongrain size. The spread event originated by a pioneer nucleation eventpropagates until it reaches a volume, vsp, Equation 1. As a result, vsp, isassumed to remain constant throughout the transformation. Therefore,the extended volume of spread can be calculated by 0. The calcuThe actual t0 will be larger than t0,lower because ni,Tvlated values of t0,lower are given in Table 2. In diffusional reactions, anucleation event generates a very small volume of the product phasewhich then grows relatively slowly. However, in martensitic reactions,once a nucleus is activated a plate grows nearly instantaneously to itsfinal size. As a consequence, a finite volume fraction of martensiteand also of partially transformed grains become instantaneouslygreater than zero at t t0 whereas the volume fraction of martensiteis equal to zero for t t0.5. DiscussionAs early as 1887, Thomson (Lord Kelvin)17 proposed that thetruncated octahedron or tetrakaidecahedron network would providea space-filling arrangement of similar cells of equal volume with aminimal interface area. This problem is still of interest to this day18.In Metallurgy one often adopts Kelvin’s network as an approximationfor the polycrystalline structure. We will use such an approximationhere to offer a geometric interpretation of the values of γ found fromthe above analysis.(12)defining1000(13)(14)introducing equation 14 into equation 2 obtains(15)Eqution 15 can be rearranged and be put in a form more convenientfor comparison with experimental data, see below,800ln (1/(1-Vvg))/(Gq) (mm )and inserting Equation 13 into Equation 12 gives6004002000(16)Figure 3 shows that plotting the left hand side of Equation 16 as afunction of time results in straight lines of the formIsotermalFe-28 wt. (%) Ni-3 wt. (%) Mnd 0.31 mmFitted R 0.98d 0.79 mmFitted R 0.98050010001500200025003000Time (s)Figure 3. Isothermal martensite experimental data from a Fe-23. wt. (%)Ni‑2.8 wt. (%) Mn alloy obtained by Ghosh10,11 plotted after Equation 17.The correlation coefficients, R, are also shown.
Vol. 11, No. 1, 2008107Formal Analysis of Isothermal Martensite SpreadThe basic unit of Kelvin’s network is the truncated octahedron thatis depicted in Figure 4. It is a polyhedron possessing fourteen faces,eight hexagonal faces and six square faces. In order to fill space theymust be packed in a body centered cubic structure. The BCC unit cellwith nine truncated octahedra is shown in Figure 5. The polyhedronlocated at the center of the BCC cell shares a hexagonal face witheach polyhedra located at the vertices. A full cluster consisting of 15truncated octahedral is shown in Figure 6. The other six polyhedral areFigure 4. Truncated octahedron or tetrakaidecahedron, the basic unit for aspace-filling network, proposed by Thomson17. This polyhedron has 14 faces:8 hexagons and 6 squares.attached in such a way that they share a square face with the polyhedronlocated at the center of the BCC cell. These six polyhedra occupy thecenter of adjacent BCC cells. If all polyhedra on these adjacent
Vol. 11, No. 1, 2008 Formal Analysis of Isothermal Martensite Spread 105 ship S v m 2/λ. The values of q were calculated from λ assuming that parent austenite grains can be approximated by .
Martensitic transformation via quenching Martensite: crystal structure and properties The isothermal decomposition of austenite. The TTT (time-temperature transformation) diagram. Formation pearlite and bainite. Decomposition of austenite on continuous cooling (CCT diagram). Formation of martensite and the martensite lines.
The complete isothermal transformation diagram for an iron-carbon alloy of eutectoid composition. A: austenite , B: bainite , M: martensite , P: pearlite TTT diagram gives 1- Nature and type of transformation . 2- Rate of transformation. 3- Stability of phases under isothermal transformation conditions.
Overview of Isothermal Titration Calorimetry ITC experimental design Data analysis Troubleshooting . What is isothermal titration calorimetry (ITC) A direct measurement of the heat generated or . calorimetry is a direct readout . 19 GE Title or job number 11/2/2012 Stoichiometry
ASTM C 1702 – Heat of hydration using isothermal calorimetry Heat of Hydration. is the single largest use of isothermal calorimetry in the North American Cement industry Other major applications include . Sulfate optimization . and . admixture compatibility Several Round Robins in North America and Europe on Heat of Hydration .
Isothermal Community College provides educational and employment opportunities without regard to veteran status, race, color, religion, age, sex, national origin, or disability. Isothermal Community College is committed to this polic
Isothermal transformation Diagram The Iron-Carbon system is very significant for industrial applications, and has been widely studied. The formation of pearlite from austenite has been extensively investigated under both isothermal transformation and continuous cooling conditions – Isothermal transformation: samples are held at
Hardness in isothermal cooling time function at a temperature t pi 300 and 400 C for variant I and II is presented in Table 2. t Table 2. The results of hardness measurements Austenitizing temperature 8 tγ 1), t γ' 2), 0C Isothermal transforma tion temper-ature t pi, 0C Variant of heat treat-ment Isothermal transfor-mation time τ pi, min .
Centre for Comparative and Clinical Anatomy, University of Bristol, Southwell Street, Bristol, BS2 8EJ. Receipt of these documents is a condition of our acceptance. If we do not receive these documents within 14 days of registration of the death we will be required to decline the bequest. At the time of donation the next of kin or executor will be asked to complete form ‘Instructions for the .