Cell-free Protein Synthesis: The State Of The Art
Biotechnol Lett (2013) 35:143–152DOI 10.1007/s10529-012-1075-4REVIEWCell-free protein synthesis: the state of the artJames W. WhittakerReceived: 6 September 2012 / Accepted: 10 October 2012 / Published online: 21 October 2012Ó Springer Science Business Media Dordrecht 2012Abstract Cell-free protein synthesis harnesses thesynthetic power of biology, programming the ribosomal translational machinery of the cell to createmacromolecular products. Like PCR, which usescellular replication machinery to create a DNAamplifier, cell-free protein synthesis is emerging as atransformative technology with broad applications inprotein engineering, biopharmaceutical development,and post-genomic research. By breaking free from theconstraints of cell-based systems, it takes the next steptowards synthetic biology. Recent advances in reconstituted cell-free protein synthesis (Protein synthesisUsing Recombinant Elements expression systems) arecreating new opportunities to tailor the reactions forspecialized applications including in vitro proteinevolution, printing protein microarrays, isotopic labeling, and incorporating nonnatural amino acids.Keywords Cell-free translation Proteinengineering Protein synthesis PURE expression Synthetic biologyJ. W. Whittaker (&)Division of Environmental and Biomolecular Systems,Institute for Environmental Health, Oregon Healthand Science University, 20000 N.W. Walker Road,Beaverton, OR 97006-8921, USAe-mail: jim@ebs.ogi.eduIntroductionProteins represent the ultimate expression of biochemical function: as enzymes, they perform exquisitely selective chemical reactions; as receptors, theyrecognize specific partners in signaling pathways thatintegrate and regulate metabolism; as transporters,they control trafficking across cell membranes; and asmacromolecular building blocks, they form the scaffolding of cell architecture. In biotechnology, proteinsare important not only for their remarkable catalyticpower, but also for their unique physical properties,and as tools of discovery in the development of newtherapeutics. As a result, the production of pureproteins has itself become an important goal, drivingthe evolution of both chemical and biochemicalmethods for protein synthesis. Conventional solidphase chemical synthesis provides the ultimate controlover molecular structure, but is generally restricted tosmaller peptides (\40 residues) (Nilsson et al. 2005),limiting its ability to meet the need for a broad range ofproteins for industrial and research applications.Alternatively, several decades of biochemical researchhas expanded and refined methods for recombinantprotein production based on ribosomal protein synthesis. The protein expression field has traditionallybeen dominated by cell-based expression systems,including both conventional (Escherichia coli, Saccharomyces cerevisiae) and non-conventional (e.g.,Pichia pastoris, Thermus thermophilus) host organisms that have been developed as ‘cell factories’.123
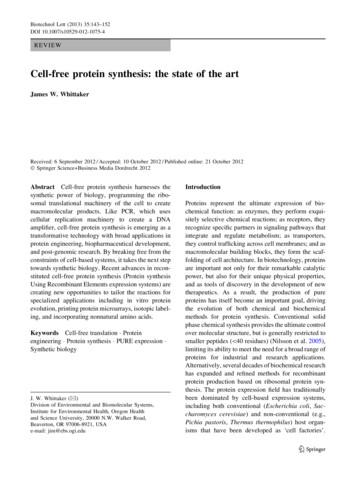
144However, cell-free protein synthesis is rapidly emerging as an important complementary approach, rivalingcell-based protein production in terms of both convenience and scalability, and becoming widely recognized as a valuable tool for protein engineering(Hodgman and Jewett 2012; Kai et al. 2012; Swartz2012).Cell-free protein synthesis is not a new concept.Protein synthesis in cell-free extracts helped to crackthe genetic code and has been extensively used todissect ribosomal protein biosynthesis, providing aspecialized niche for the development of these methods. However, advances in cell-free protein synthesisin recent years, particularly the ability to reconstituteprotein synthesis from well-defined, purified components, has transformed the approach from a specializedanalytical tool to a powerful preparative method withbroad applicability. Cell extract—or lysate-derivedsystems for protein synthesis—continue to be exploitedand optimized, with the traditional wheat germ, rabbitreticulocyte and E. coli lysates being extended withLeischmanii (Kovtun et al. 2011), Thermus (Zhou et al.2012) and even human (Mikami et al. 2008) cell-freesystems. Systems based on reconstituted highly purifiedcomponents (PURE expression systems) have significantly extended the range of applications and will be theprimary focus of this mini-review, which surveys recentadvances and emerging applications of cell-free proteinsynthesis.Biotechnol Lett (2013) 35:143–152system that can be programmed for protein synthesisusing a variety of DNA templates (Fig. 1). The PUREcell-free protein synthesis system has been commercialized (e.g., PURESYSTEM, Cosmo Bio, Tokyo,Japan; PURExpress, New England Biolabs, Beverly,MA, USA), making it available for a variety of laboratory research applications.The advantages of the PURE systems includereduced levels of contaminating proteases, nucleases,and phosphatases, greater reproducibility resultingfrom more defined chemistry, and the flexibility of amodular system. Metabolic side reactions that depletethe amino acid pool in cell extracts can be entirelyavoided. In addition, the 6 9 His affinity tags associated with the PURE components can be utilized in‘reverse purification’ of products, extracting thetagged proteins by metal affinity chromatography.PURE expression systemsThe Protein synthesis Using Recombinant Elements(PURE) approach to cell-free protein synthesis is basedon modular reconstitution of the translational machinery of the cell from affinity purified protein components (Shimizu et al. 2005; Shimizu and Ueda 2010;Wang et al. 2012), including initiation factors (IF1,IF2, IF3), elongation factors (EF-Tu, EF-Ts, EF-G),release factors (RF1, RF2, RF3), ribosome recyclingfactors, 20 aminoacyl tRNA synthetases, methionyltRNA formyltransferase and pyrophosphatase, allbearing a 6 9 His tag. These recombinant componentsare combined with ribosomes and tRNAs isolated fromspecially engineered E. coli strains, together with all ofthe necessary NTPs and amino acids, an ATP-generating catalytic module and recombinant T7 RNApolymerase (RNAP), creating a self-contained reaction123Fig. 1 PURE protein translation system. The PURE expressionsystem is programmed by a DNA template and includes atranscription module, an energy-coupling module for NTPregeneration, aminoacyl tRNA synthetases and tRNAs, and atranslation module comprised of ribosomes, elongation factors,and release factors that are integrated to perform peptideelongation and macromolecular synthesis
Biotechnol Lett (2013) 35:143–152Because it is modular, the PURE system supports avariety of modifications for specialized applications,including ribosome display and site-selective incorporation of nonnatural amino acids, described in moredetail below. Both cell extracts and the components ofthe PURE system can be modified to mimic themacromolecular crowding of the interior of a cell (Geet al. 2011).ATP-regeneration moduleRibosomal protein synthesis is an energy-intensiveprocess, requiring approximately 4 ATP equivalents/peptide bond (including 2 ATP equivalents/residue foramino acid activation, 1 GTP/residue for transfer ofthe charged aminoacyl-tRNA to the A site of theribosome, and 1 GTP/residue for translocation of theribosome through coupling to EF-G) (Calhoun andSwartz 2007; Kim and Kim 2009). Consequently, thechoice of ATP-regenerating system is a critical factorfor cell-free protein synthesis. A variety of ATPregenerating reactions have been explored to achievesustained, stable production of ATP while avoidingaccumulation of inorganic phosphate, which inhibitstranslation by binding Mg2? that serve as essentialcofactor in many nucleotide-dependent reactions,including protein synthesis. The choice of ATPregeneration module determines both the rate andduration of active protein synthesis.In the PURE systems, all enzymes required to forma complete in vitro catalytic pathway must be includedto regenerate ATP in one or more substrate-levelphosphorylation steps. High phosphoryl-group transfer potential substrates (creatine phosphate, phosphoenolpyruvate (PEP), 3-phosphoglycerate) can be useddirectly as phosphate donors, although they arerelatively expensive. Alternatively, non-phosphorylated substrates may be used, with the phosphateentering from ATP (lowering the overall yield) or asinorganic phosphate. Adenylate kinase and nonspecific nucleotide diphosphate kinase must also beincluded to catalyze phosphate exchange within thepool of ribonucleotides. These two enzymes equilibrate the phosphorylation state of all four nucleotides,and couple ATP regeneration to formation of GTP,which is required for delivery of charged tRNAand for translocation of the ribosome during proteinsynthesis.145Phosphate donorsThe simplest ATP regeneration system uses highgroup transfer potential phosphorylated compounds topower the translational machinery. While its simplicity is an advantage, this type of system invariably leadsto accumulation of inorganic phosphate as an endproduct. In the original formulation of the PUREtranslation system, creatine phosphate was added as arelatively stable source of high group transfer potentialphosphate, and the enzyme creatine kinase was used tocatalytically regenerate ATP from ADP (Fig. 2). Theorganic end product, creatine, is relatively innocuousand does not appear to interfere with protein synthesis.However, because of the 1:1 stoichiometry of thephosphoryl transfer reaction, relatively large amountsof creatine phosphate substrate are required, and oneequivalent of inorganic phosphate is formed in eachreaction cycle. The accumulation of phosphate can bedelayed by including glucose as a secondary energysource (Kim et al. 2007).The glycolytic intermediate, 3-phosphoglycerate,supports substrate-level phosphorylation in the presence of three enzymes of the glycolytic pathway(phosphoglyceromutase, enolase and pyruvate kinase).Once again the 1:1 stoichiometry for ATP production isa limiting factor. Another simple ATP-regenerationmodule is based on acetate kinase-catalyzed formationof ATP from ADP and acetyl phosphate, which isreadily available (Ryabova et al. 1995), althoughformation of an organic acid (acetic acid) as an endproduct may interfere with protein synthesis (Wang andZhang 2009). Polyphosphate, a linear polymer ofphosphoric acid that occurs naturally in cells, can alsoserve as a phosphate donor for ATP regeneration.However, as in the other examples described above,chelation of divalent metal ions by inorganic phosphateis a complicating factor.Reconstituted pathwaysSome of the problems associated with ATP-regenerationbased on simple phosphate donors can be resolved byreconstituting extended metabolic pathways, increasingthe yield of ATP and recycling the inorganic phosphate.So far, approaches based on well-characterized cellularmetabolism (including glycolysis and oxidative phosphorylation) have mainly been applied in cell extracts,although reconstitution of extended metabolic pathways123
146Fig. 2 ATP regeneration pathways for cell-free protein synthesis. Commonly used reaction sequences (Cre-P (Creatinephosphate); PANOxSP (PEP, Amino acids, NAD?, Oxalic acid,Spermidine and Putrescine)) are indicated. The enzymes requiredfor these reactions are: (1) creatine kinase; (2) hexokinase; (3)phosphofructokinase; (4) aldolase ? triose phosphate isomerase ? glyceraldehydes-3-phosphate dehydrogenase ? 3-phosphoglycerate mutase ? enolase; (5) pyruvate kinase; (6)pyruvate dehydrogenase; (7) phosphate acetyltransferase; (8)acetate kinase; (9) lactate dehydrogenase; (10) pyruvate oxidase;(11) phosphorylase; (12) phosphoglucomutaseBiotechnol Lett (2013) 35:143–152A number of variations on this theme have beendescribed. The PANOxSP [PEP, Amino acids, NAD?,Oxalic acid, Spermidine and Putrescine] systemutilizes PEP as a simple phosphate donor but extendsthe biochemical processing through the pyruvatedehydrogenase complex, and recycles one equivalentof inorganic phosphate, with a net yield of two ATPfor each PEP (Calhoun and Swartz 2007) (Fig. 2).Another system, called cytomim (cytosolic mimicry),effectively reconstitutes oxidative phosphorylation,consuming simple organic acids (e.g., succinate) andusing inverted respiratory membranes to generate ATP(Jewett and Swartz 2004; Jewett et al. 2008).A particularly interesting method of ATP-regeneration has recently been described that involves the use ofglucose storage polymers (maltodextrin or soluble starch)as a reservoir of chemical free energy (Wang and Zhang2009; Kim et al. 2011). This approach is related to thereconstituted glycolysis system described above but isextended to include phosphorolytic cleavage of thestorage polymer. There are four major advantages of thisscheme: (1) it allows use of an inert and inexpensivemaltodextrin or starch substrate, (2) it provides a‘pacekeeper’ reaction that moderates ATP production,(3) it generates a high phosphoryl group transfer potentialintermediate (glucose 1-phosphate) in situ, and (4) itcompletely recycles inorganic phosphate. When combined with the reactions of glycolysis and the PANOxpathway, the reactions have a theoretical yield of fourATP per glucose. This appears to be the most efficientenergy coupling module currently available. Development of reaction modules to drive cell-free proteinsynthesis is an active area of research, and the possibilitiesare far from being exhausted.Continuous exchangein vitro from purified components is also possible(Stevenson et al. 2012).The glycolytic reaction sequence is well-suited to ATPregeneration in the cell-free system, since the reactions donot require O2 and the presence of two substrate-levelphosphorylation steps enhances the yield of ATP. Whenglucose is used, two ATP equivalents are required forsubstrate activation but four ATP are formed, and twoequivalents of inorganic phosphate are recycled. Onedrawback of this scheme is that the redox stoichiometry ofglycolysis results in a net production of NADH as an endproduct (Kim et al. 2008).123Some of the limitations inherent in closed system(batch) reaction scheme described above can beovercome in a continuous exchange open-systemformat allowing substrates to be replenished duringthe reaction (Yin et al. 2012). For example, filtration ordialysis can be used to continuously supply ATP as itis consumed and to remove inorganic phosphate as it isformed. This strategy is very effective at extending theduration of active translation in small scale cell-freesystems, but is not as scalable. Other strategies, inwhich the open-system character is restricted to pHcontrol, have been used to extend cell-free protein
Biotechnol Lett (2013) 35:143–152synthesis to the industrial scale resulting in a highyield of a disulfide-bonded protein product (700 mg/l)(Zawada et al. 2011).Transcription moduleCell-free protein synthesis is programmed by additionof a DNA template, formed from either closed circularvector DNA or a linear PCR product. Transcription isperformed by recombinant phage T7 RNAP, generating the mRNA upon which the ribosomal translationmachinery acts (Beckert and Masquida 2011). T7RNAP is a relatively simple, single-subunit polymerase with high promoter specificity and transcriptionalfidelity (Sousa and Mukherjee 2003).The minimum requirements for the DNA templateinclude a 50 -untranslated region (UTR) comprisinga strong T7 promoter sequence to support a hightranscription rate, a Shine-Delgarno sequence thatserves as the ribosome entry point, and a 30 -UTR thatincludes an efficient translation termination codon(e.g., TAA), followed by six or more nucleotides(Shimizu and Ueda 2010). An epsilon (Enhancer ofProtein Synthesis Initiation) sequence may be added tothe 50 -UTR to improve translation, and a T7 terminatorin the 30 -UTR improves efficient release and recyclingof the ribosome. This simple transcription unit iseasily prepared by two-step PCR, allowing virtuallyany coding sequence to be assembled togetherwith promoter and terminator elements for cell-freesynthesis.Transcription and translation processes can bearranged as either linked or coupled reactions. Linkedtranscription/translation implies a two-step sequentialprocess, where the transcript is formed first. Incontrast, coupled transcription/translation impliessimultaneous synthesis of mRNA and protein withinan extended polysome complex. Cell-free proteinsynthesis based on prokaryotic components generallyinvolves a coupled reaction system (Fig. 1).It is possible to extend cell-free synthesis tosimultaneous production of multiple polypeptides ina single reaction mixture, since addition of multipletemplates results in the parallel synthesis of distinctproteins. This approach can be used to assemblecomplex multicomponent proteins, as demonstratedby the successful cell-free synthesis of the heterotrimeric core of Paracoccus denitrificans cytochrome147c oxidase in an E. coli extract (Katayama et al. 2010).An alternative strategy for multigene expression frompolycistronic constructs has been demonstrated forproduction of up to five distinct protein products froma single ‘BioBrick’ plasmid template (Du et al. 2009).Sequential synthesis is also possible: by immobilizingtemplate DNA on magnetic microbeads, cell-freeprotein synthesis can be arbitrarily reset and reprogrammed, an example of artificial gene circuits (Leeet al. 2012).Typical protein synthesis reactions are driven bytranscription from sub-pmol quantities of template,and increasing improvements in the efficiency oftranscriptional processing has brought the technologynear the single-molecule limit of template sensitivity.In a recent example, green fluorescent protein wasproduced at quantized expression levels from 1 to 2copies of template in picoliter volume reactionmicrochambers (Okano et al. 2012). This level ofsensitivity is important for a variety of nanoscaleprocessing applications, including printing proteinmicroarrays.Addition of a species-independent translationsequences (SITS) in the 50 -UTR of the templateeliminates species barriers to cell-free translation. Thisuniversal adapter element relaxes secondary structurein the transcript and facilitates assembly of thetranslation complex in yeast, wheat germ, insect cell,rabbit reticulocyte and E. coli translation systems,opening up new possibilities for cell-free proteinexpression (Mureev et al. 2009).tRNA aminoacylation moduleAmino acids are activated for peptide bond formationby amino acyl tRNA synthetases that are specific forthe amino acid substrate and the cognate set of tRNAs,covalently linking the residue to the 30 -terminaladenosine of the tRNA (Ling et al. 2009). Formationof inorganic pyrophosphate as a by-product duringamino acid activation requires inorganic pyrophosphatase and adenylate kinase to be present to drive thereaction and recycle AMP.Protein synthesis requires that all 20 amino acidsare present in amounts super-stoichiometric with theamount of protein to be formed, in addition to catalyticamounts of all 20 amino acid tRNA synthetases andthe full complement of tRNAs. In the PURE system123
148the synthetases are affinity purified recombinantproducts, and tRNAs are isolated from specialE. coli strains (Shimizu and Ueda 2010). The modulardesign of the PURE translation system makes itpossible to supplement with rare tRNAs to compensatefor codon bias in the template.Modifying the composition of the amino acidmixture can be used to control the translation process.For example, using a drop-out mixture lacking oneamino acid will result in ribosome stalling or pausing,allowing expression to be synchronized. Substitutionwith isotopomers facilitates isotopic labeling (withstable isotopes or radionuclides) while avoiding isotopedilution and scrambling resulting from unavoidablemetabolic processing in cell-based systems (Ozawaet al. 2005; Su et al. 2011; Yokoyama et al. 2011).These approaches also permit efficient incorporation ofSe-methionine into proteins without the toxic sideeffects that can compromise in vivo selenium labeling(Kigawa et al. 2002). Insertion of photochemicallyactive analogs that are recognized by the native E. colitRNA aminoacylation machinery (e.g., photo-leucineand photo-methionine (Suchanek et al. 2005)) is alsoexpected to be straightforward. Alternatively, bioorthogonal pairs of suppressor tRNA/cognate aminoacyltRNA synthetase can be added to the system to directthe incorporation of nonnatural amino acids into theprotein product (Goerke and Swartz 2009; Ozawa et al.2012), or aminoacylating ribozymes (flexizymes) canbe used to expand the genetic code (Goto et al. 2011;Goto and Suga 2012) (see below).Peptide synthesis modulePeptide synthesis is catalyzed by ribosomes, large(megadalton) ribonucleoprotein complexes comprisedof more than 50 proteins and three RNAs thatrepresents about 30 % of the mass of rapidly growingbacterial cells (Bremer and Dennis 2008). Although inprinciple a small, catalytic amount of ribosomes couldbe continuously recycled during cell-free proteinsynthesis, in practice the yield of protein tends to beroughly proportional to the quantity of ribosomes,which therefore represent one of the main componentsof the reaction mixture. Because of their abundance,unmodified native ribosomes may be easily isolatedfrom E. coli. However, an engineered strain of E. coliproducing His-tagged ribosomes is now available123Biotechnol Lett (2013) 35:143–152thereby allowing a one-step purification of activeribosomes (Ederth et al. 2009) that lend themselves tothe ‘reverse purification’ strategy described above.The PURE systems also provides control over theavailability of release factors, facilitating applicationslike ribosome display (see below) by promotingstalling of the ribosome on the mRNA, or incorporation of nonnatural amino acids by suppressor tRNAs.Posttranslational processingThe modular design of the cell-free expression systemprovides a high degree of control over posttranslational processing events. One of the most widelyappreciated advantages of the cell-free system is thepossibility of directing insertion of an integral membrane protein product into a lipid structure, avoidingproblems that are often encountered in high-levelexpression of membrane proteins in cellular systems(Schneider et al. 2010). Expression of integral membrane proteins in living cells can be problematic as aresult of toxic side-effects relating to membranedisruption. Cell-free synthesis of integral membraneproteins has been accomplished utilizing a varietyof lipid structures, including liposomes, micelles,bicelles and nanodiscs (Lyukmanova et al. 2012).Bacteriorhodopsin synthesized in a cell-free systemhas been cotranslationally inserted into giant liposomes, where its photochemical proton pumpingfunction could be demonstrated (Kalmbach et al.2007). Nanodisc technology appears to be particularlywell-suited to marrying cell-free synthesis with protein structural studies, since the nanodisc is structurally well-defined (Bayburt and Sligar 2010).Correct folding of the recombinant protein can beassisted by supplementing the basic PURE systemwith molecular chaperones to enhance the efficiencyof protein folding, helping polypeptides navigate thefolding funnel and reach their native conformationalstate (Shimizu et al. 2005; Ueda 2008). Interestingly,even when the cell-free synthesis protein product isinsoluble, it tends to be more readily solubilized andre-folded than proteins that have been recovered frominclusion bodies in cell-based expression systems(Swartz 2012). The formation of disulfide bonds isanother important posttranslational processing stepthat can be problematic in prokaryotic expressionsystems. In cell-free protein synthesis, disulfide bond
Biotechnol Lett (2013) 35:143–152formation has been shown to be enhanced by additionof a glutathione redox buffer that facilitates disulfideexchange (Goerke and Swartz 2008; Knapp et al.2007).Applications of cell-free protein synthesisCell-free protein synthesis is an enabling technologythat has the potential to transform many aspects ofbiotechnology. Its value has already been demonstrated in production of ‘difficult’ proteins that may betoxic to conventional cell protein factories, and newapplications are emerging in protein engineering, drugdiscovery and synthetic biology.Printing protein arraysProtein microarrays have emerged as a promisingplatform for high-throughput screening in postgenomicbiomedical research, permitting massively parallelfunctional analysis of complete proteomes and providing an important tool for vaccine development andpersonalized medicine (Berrade et al. 2011). Cell-freeprotein synthesis is a crucial link between the established technology of DNA microarrays and proteinarrays allowing multiplexed, robotic processing to beused to print protein arrays from gene chips. Cell-freeexpression based in situ protein microarrays can beproduced by a variety of methods that take advantage ofthe programmability of ribosomal protein synthesis bytranscripts formed from either linear DNA (generatedby PCR) or circular plasmid vectors: PISA (proteinin situ array), DAPA (DNA array to protein array),NAPPA (nucleic acid programmable protein array) andTUS-TER mediated affinity labeling differ in thedetails of template construction and affinity capturestrategy (Nand et al. 2012).Ribosome displayRibosome display is an approach to protein functionalanalysis that can be used as a platform for proteinengineering that is based on the stability of theribosomal translation complex (comprised of mRNA,ribosome, and nascent polypeptide) in the absence ofrelease factors. This translation complex provides aphysical link between gene and protein that can beutilized in a recursive cycle of functional selection and149mutagenesis to drive protein evolution (Plückthun2012). The modular composition of the PURE cellfree system is especially well-suited to this application(Ohashi et al. 2007; Ueda et al. 2010), because itallows release factors to be omitted as a rational choicein the preparation and can accommodate incorporationof nonnatural amino acids (Watts and Forster 2012).Isotopic labelingLabeling of recombinant proteins with stable isotopesor radionuclides is important for a variety of applications, including biological NMR and tracer experiments. When proteins are expressed in living cells orcell extracts, labeling is complicated by metabolicprocesses that dilute isotope or scramble labelingpatterns as a result of molecular transformations,although strategies have been devised to suppressthese side-reactions (Su et al. 2011; Yokoyama et al.2011; Matsuda et al. 2007). In contrast, the severelyedited metabolic map in PURE cell-free proteinsynthesis may be expected to eliminate many of theseproblems, because the interfering enzymes and metabolites are not present (Ozawa et al. 2005).Incorporation of nonnatural amino acidsExpansion of the genetic code is perhaps the mostsignificant development in ribosomal protein synthesissince biochemistry based on the canonical set of 20amino acids evolved 3 billion years ago, adding newdimensions to protein engineering (Liu and Schultz2010). The approach is conceptually simple, using abioorthogonal cognate pair of tRNA and aminoacyltRNA synthetase to suppress nonsense or frameshiftmutations, incorporating nonnatural amino acids intothe growing polypeptide and thereby creating achemical toolbox for protein engineering. By systematic evolution of cognate tRNA/aminoacyl tRNAsynthetases, more than 50 distinct nonnatural aminoacids have been incorporated into proteins in this way,adding fluorescent tags or reactive groups that contribute novel chemistry or functionality in the proteinproduct (de Graaf et al. 2009; Hartman et al. 2007).While the majority of this work has been performedin vivo, cell-free expression systems offer someimportant advantages (Goerke and Swartz 2009;Ozawa et al. 2012). For example, cellular systems aresusceptible to toxic side-effects of non-natural amino123
150acids in cellular proteins or recombinant products. Incontrast, cell-free systems avoid toxicity issues. Further, the cell-free system can be more easily tailored toaccommodate unusual side chain structures by modifying the components of the translation machinery.Mutation of EF-Tu has already been shown to improvethe efficiency of incorporating nonnatural amino acidswith bulky side chains in the PURE system (Doi et al.2007). The well-defined chemistry of the PURE systemalso lends itself to customization in terms of the choiceof tRNAs and aminoacyl tRNA synthetases. Nonnatural amino acid chemistry can be introduced into theprotein product simply by adding the bioorthogonalcomponents. The effectiveness of this approach hasalready been demonstrated in cell-free extracts bypreparation of p-propargyloxyphenylalanine-containingproteins that can be crosslinked to form bioconjugatesvia click chemistry (Bundy and Swartz 2010). Selective omission of the RF1 release factor can enhancesuppression by the bioorthogonal tRNA, allowingincorporation of nonnatural amino acids at multiplesites (Johnson et al. 2011; Loscha et al. 2012).Ribozyme technology has recently been developedas an alternative strategy for expanding the geneticcode. Flexizymes are small catalytic RNAs thatsupport acylation of tRNA with virtually any aminoacid analog (Goto et al. 2011; Goto and Suga 2012).Flexizymes can be subjected to rapid in vitro molecular evolution to select for optimized performance,and the lack of membrane barriers in the cell-freesystem makes it the ideal platform for both selectionand application of flexizyme technology.Comparison of platforms for protein synthesisRibosomal protein synthesis (both cellular and cellfree) is characterized by exceptionally high fidelity,with an amino acid misincorporation frequency ofapprox. 0.01 %, even at high elongation rates (20residues/s) (Yadavalli and Ibba 2012). This makes itpossible t
Protein synthesis in cell-free extracts helped to crack the genetic code and has been extensively used to dissect ribosomal protein biosynthesis, providing a specialized niche for the development of these meth-ods. However, advances in cell-free protein synthesis in recent years, particularly the ability to reconstitute protein synthesis from .
May 02, 2018 · D. Program Evaluation ͟The organization has provided a description of the framework for how each program will be evaluated. The framework should include all the elements below: ͟The evaluation methods are cost-effective for the organization ͟Quantitative and qualitative data is being collected (at Basics tier, data collection must have begun)
Silat is a combative art of self-defense and survival rooted from Matay archipelago. It was traced at thé early of Langkasuka Kingdom (2nd century CE) till thé reign of Melaka (Malaysia) Sultanate era (13th century). Silat has now evolved to become part of social culture and tradition with thé appearance of a fine physical and spiritual .
On an exceptional basis, Member States may request UNESCO to provide thé candidates with access to thé platform so they can complète thé form by themselves. Thèse requests must be addressed to esd rize unesco. or by 15 A ril 2021 UNESCO will provide thé nomineewith accessto thé platform via their émail address.
̶The leading indicator of employee engagement is based on the quality of the relationship between employee and supervisor Empower your managers! ̶Help them understand the impact on the organization ̶Share important changes, plan options, tasks, and deadlines ̶Provide key messages and talking points ̶Prepare them to answer employee questions
Dr. Sunita Bharatwal** Dr. Pawan Garga*** Abstract Customer satisfaction is derived from thè functionalities and values, a product or Service can provide. The current study aims to segregate thè dimensions of ordine Service quality and gather insights on its impact on web shopping. The trends of purchases have
Chính Văn.- Còn đức Thế tôn thì tuệ giác cực kỳ trong sạch 8: hiện hành bất nhị 9, đạt đến vô tướng 10, đứng vào chỗ đứng của các đức Thế tôn 11, thể hiện tính bình đẳng của các Ngài, đến chỗ không còn chướng ngại 12, giáo pháp không thể khuynh đảo, tâm thức không bị cản trở, cái được
protein:ligand, K eq [protein:ligand] [protein][ligand] (1) can be restated as, K eq 1 [ligand] p 1 p 0 (2) where p 0 is the fraction of free protein and p 1, the fraction of protein binding the ligand. Assuming low protein concentration, one can imagine an isolated protein in a solution of Nindistinguishable ligands. Under these premises .
An Offer from a Gentleman novel tells Sophie’s life in her family and society. Sophie is an illegitimate child of a nobleman having difficulty in living her life. She is forced to work as a servant because her stepmother does not like her. One day, Sophie meets a guy, a son of a nobleman, named Benedict. They fall in love and Sophie asks him to marry her legally. Nevertheless Benedict cannot .