CHAPTER 4 TEST: Atoms, Atomic Theory And Atomic Structure
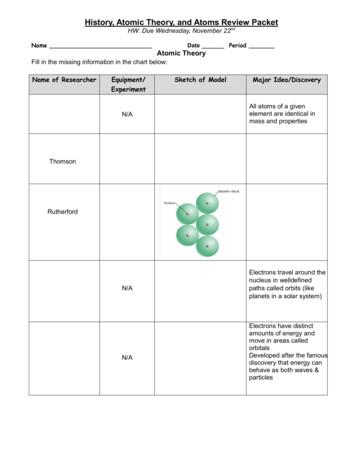
CHAPTER 4 TEST:Atoms, Atomic Theory and Atomic tusThomsonC.F.RutherfordSchrodingerdate:1.Greek thinker; called nature’s basic particle an atom, based on the Greek word“atomos” which means “indivisible”. Did not have evidence that atoms existed.2.Danish physicist who in 1913 suggested that the energy of each electron was related to theelectron’s path around the nucleus.3.English schoolteacher; Like Democritus, he believed that atoms are the fundamental units ofmatter and are indivisible. Atoms of the same element are alike; Was able to base his theoryon experimental evidence. Parts of his theory included the Law of Multiple Proportions (usedto form compounds) and The Law of Conservation of Mass.4.New Zealand scientist; Famous for gold-foil experiment; Concluded that the atoms positivecharge is concentrated in the nucleus and that the nucleus is very small in relation to the atomand very dense. The negative electrons orbit the positively charged nucleus much like planetsorbit the sun.5.Austrian scientist; Treated electrons like waves; In 1926 credited with discovering theELECTRON CLOUD MODEL.6.English physicist; Responsible for discovering electron using cathode-ray experiment; Thismeant that atoms could be divided into smaller parts. Also credited with “plum puddingmodel” where electrons were like plums embedded in a positively charged “pudding”.MULTIPLE CHOICE.In the space provided, write the letter of the term or phrase that best completes each statement or best answerseach question.1. Democritus’s original atomic theory was revised because ita. claimed matter is made of atoms.c. explained what electrons are.b. claimed atoms could be divided.d. did not have a scientific basis.2.Dalton’s atomic theory stated that every element was made of atoms that could not besubdivided, atoms of the same element are alike, anda. atoms are made of protons, neutrons, and electrons.b. the nucleus is the center of the atom.c. atoms of different elements could form to join compounds.d. atoms are constantly in motion.3. Who determined that atoms could be divided?a. Democritusc. Daltonb. Thomsond. Rutherford
4. Thomson made his discovery about the atom during an experiment usinga. thermal energy.c. cathode rays.b. kinetic energy.d. X rays.5. Thomson is responsible for discovering that an atom containsa. electrons.c. anodes.b. molecules.d. a nucleus.6. In atomic model, negative electrons orbit the positively chargednucleus.a. Dalton’sc. Rutherford’sb. Thomson’sd. Democritus’s7. Whose model determined that an atom’s positive charge is concentrated in the atom’s center?a. Rutherford’sc. Democritus’sb. Dalton’sd. Thomson’s8.According to Rutherford’s model of the atom, electrons behave likea. planets orbiting the sun.c. light energy in a vacuum.b. waves on a vibrating string.d. planets rotating on their axes.9.What did Rutherford learn about the atom in the gold-foil experiment?a. Atoms have electronsb. Atoms have a nucleusc. Atoms have negative charge embedded in a sphere of positive charge.d. The nucleus is most of the atom’s volume10. Which statement is false according to Bohr’s model of the atom?a. Electrons cannot be between energy levelsb. Electrons orbit the nucleusc. An electron’s path is not known exactlyd. Electrons exist in energy levels11. Unlike the modern model of the atom, Bohr’s model states thata. electrons move in set paths around the nucleus of an atom.b. atoms cannot be divided into smaller parts.c. electrons behave like waves.d. electrons contain orbitals.12. According to the modern model of the atom,a. moving electrons form an electron cloudb. electrons and protons circle neutronsc. neutrons have a positive charged. the number of protons for a given element varies13. A subatomic particle that has a negative charge is called a(n)a. molecule.c. element.b. electron.d. compound.14. What is an atom’s nucleus made of?a. protons and neutronsc. only neutronsb. only protonsd. anodes
15. The charge of an atom isa. positive.b. neutral.c. negative.d. unbalanced.16. Which statement about the atom’s nucleus is correct?a.The nucleus is made of protons and neutrons and has a negative charge.b.The nucleus is made of protons and neutrons and has a positive charge.c.The nucleus is made of electrons and has a positive charge.d.The nucleus is made of electrons and has a negative charge.17. An element’s atomic number is equal to its number ofa. protons.c. valence electrons.b. neutrons.d. protons and neutrons.18. Two different isotopes of an element have differenta. numbers of neutrons.c. atomic numbers.b. numbers of protons.d. numbers of electrons.19. What is the mass number of an element that has 19 protons, 19 electrons, and 20 neutrons?a.19b.20c.39d.5820. A sodium atom, which has 11 electrons, has electron(s) in its thirdenergy level.a.0b.1c.2d.821 . The number of energy levels filled in an atom is determined h inner energy level of an atom has a maximum number of it can hold.a.protons.b.electrons.c.neutrons.d.quarks23.A certain atom has 26 protons, 26 electrons, and 30 neutrons. Its mass number is .a.26b.30c.52d.24.Oxygen’s atomic number is 8. This means that an oxygen atom hasa. eight neutrons in its nucleus.b. a total of eight protons and neutrons.c. eight protons in its nucleus.d. a total of eight neutrons and electrons.25.Which is NOT found in the nucleus of an atom?a.electronb.neutronc.proton26.d.56quarkThe element nickel has five naturally occurring isotopes. Which of the following describesthe relationship of these isotopes?a. same mass, same atomic numberb. different mass, same atomic numberc. same mass, different atomic numberd. different mass, different atomic number
27.Atoms of different elements are different because they have different numbers of what typeof .Carbon has six protons. How many valence electrons does carbon have?a.2b.4c.6d.12.29.Atoms that gain or lose electrons are ied True/False.Indicate whether the statement is true or false. If false, change the identified word or phrase to make thestatement true.1.The central core of an atom is called the nucleus.2.The region around the nucleus occupied by the electrons is called the negative zone.3.The maximum number of electrons in the second energy level of an atom is 4.4.Two isotopes of carbon are carbon-12 and carbon-14. These isotopes differ from one anotherby two protons.5.So far, scientists have confirmed the existence of six different quarks.6.The atomic number of an element is determined by its number of protons.7.According to present atomic theory, the location of an electron in an atom cannot bepinpointed exactly.8.If the atom were the size of Cleveland Browns Stadium, the nucleus would be amarble located at the 50-yard line.ENERGY LEVEL DIAGRAMS.Use the Energy Level Diagrams provided to answer each of the following.1.Which of the diagrams represent the same element?2.What is the atomic number for atom A?3.What is the atomic mass for atom C?
MORE MATCHING.In the space provided, write the term or phrase that best completes each sentence. Choose from the list below.IsotopesQuarksElectron CloudMass numberAverage Atomic MassNucleus1.Two elements with the same number of protons, but a different number ofneutrons are called .2.The positively charged center of an atom is called the .3.In the current model of the atom, the electrons are located in the .4.The is the sum of the number of protons and neutronsin an atom.5.The particles that make up protons and neutrons are called .6.is the weighted-average mass of all the known isotopesfor an element.EVEN MORE MATCHING.In the space provided, write the letter of the term or phrase that best matches each description.1. found in the outer energy level ofan atom2. where electrons are likely to be found in an atom183.ArArgon4.405.6.a. orbitalb. valence electronc. atomic numberd. symbole. namef. mass number
PROBLEMSnameUSE THE PERIODIC TABLE TO ANSWER THE FOLLOWING:1.Write the chemical symbol for each of the following elements:a. manganeseb. leadPS#date:c. carbond. uraniume. radonf. silver2.Given the information to the right from the Periodic Table:a.Find the element’s symbolb.Find the element’s atomic numberc.Find the number of protonsd.Find the number of electronse.Find the mass numberf.Find the number of neutrons13Aluminum27Complete the Bohr model below for Aluminum.g.Determine the number of valence electrons.Draw the Lewis Structure for Aluminum.
3.Fill in the following chart.ELEMENT*take this off the first Periodic Table we used in class!SYMBOLATOMICNUMBERMASSPROTONS Neutrons ElectronsNUMBER*MagnesiumFe46581414BoronCONCEPT MAPComplete the concept map below. Choose from the word bank below.QuarksElectronsProtonsNucleus12341.2.3.4.
SHORT ANSWER.1.Explain how the isotopes of an element are alike and how they are different. (2 points)2.Why do most atoms have no charge even though they are made up of positively charged protonsand negatively charged electrons? (2 points)USE THE CARTOON TOANSWER QUESTION 3.3.Explain the response ofatom A in terms of protonsand electrons. Describehow protons and electronsaffect charge. (2 points)EXTENDED RESPONSE1.Compare and contrast the model shown to the right with Bohr’s modelof an atom. (4 points) (Similarities and differences!)
2.Identify and describe the particles that make up an atom. Include their location within the atom itself.How do these particles relate to the identity of an atom? (4 points)3.Do Bohr Atomic Models like we did in class give realistic representations of atoms? Why or why not?EXTRA CREDIT.1.Name the six types of quarks.
nameOGT/STANDARDIZED TEST PRACTICEPSUse the diagram to the right to answer question 1.#date:1.The atomic number of carbon is 6, which means that carbon atoms always have 6A.ionsB.protonsC.neutronsD.valence electrons2.In his investigations of air, Henry Cavendish discovered a small bubble of leftover gas thatwould not combine with nitrogen. His observations went unnoticed until William Ramsayperformed experiments in which he obtained similar results. Ramsay recalled and repeatedCavendish’s experiments exactly to verify the results. Then, using Gustav Kirchoff’sspectroscopy technique, Ramsay was able to identify the leftover gas as the element hecalled argon. Upon further investigation, he found the elements neon, krypton and xenon.Based on this information, it can be said thatA.B.C.D.3.the combined work of Cavendish, Kirchoff and Ramsay led to the discovery of argon,neon, krypton and xenon (the noble gases).Kirchoff’s work was insignificant in the investigations leading to the discovery of argon.Ramsay violated ethical practice in science by repeating Cavendish’s experiments.Cavendish is directly responsible for the discovery of argon, but not neon, krypton orxenon.Which element does the shell model represent?
7.Which of the following diagrams represents the modern model of the atom?8.Scientists currently use radioactive isotopes in various fields. Some radioactive isotopes areused to .A.B.C.D.power lasersdevelop new antibioticsclone organismsdate ancient bones
11.According to the chart above, which solid has an atomic mass greater than 200?A.rubidiumB.cesiumC.tantalumD.thorium12.These pictures show different models of the atom proposed by scientists. Which of these isthe correct order, from oldest to most recent?A.B.C.D.13.T, R. Q, ST, S, R, QS, R, T, QR, S, T, QAlkali metals belong to a group of elements whose atoms have only one electron in their outerenergy level. According to this definition, which of these is an atom of an alkali metal?A.B.C.D.
CHAPTER 4 TEST:Atoms, Atomic Theory and Atomic StructurenamePSANSWER SHEET#Matching.MULTIPLE 5.5.12.19.6.6.13.20.7.14.21.22.TRUE OR FALSEMODIFIED .
More Matching.1.2.3.4.5.6.EVEN More Matching.1.2.3.4.5.6.More MULTIPLE 35.36.37.38.
Use the diagram to the right to answer question 1. _1. The atomic number of carbon is 6, which means that carbon atoms always have 6 A. ions B. protons C. neutrons D. valence electrons _2. In his investigations of air, Henry Cavendish discovered a small bub
A. 1.13 1024 atoms B. 1.48 1025 atoms C. 2.44 1022 atoms D. 3.22 1023 atoms E. 6.98 1021 atoms 50. How many atoms are in 7.12 mol of gold, Au? A. –1.18 10 23 atoms B. 244.29 10 atoms C. 8.46 1022 atoms D. 4.70 1024 atoms E. 3.34 1026 atoms 51. How many moles are
Atomic Structure Early Atomic Theories Dalton’s Atomic Theory: Each element is composed of extremely small particles called atoms. All of the atoms of 1 element are identical to each other but are different from the atoms of another element. Atoms of one element cannot be changed into atoms of a different element by chemical
Part One: Heir of Ash Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 Chapter 24 Chapter 25 Chapter 26 Chapter 27 Chapter 28 Chapter 29 Chapter 30 .
TO KILL A MOCKINGBIRD. Contents Dedication Epigraph Part One Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 Part Two Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18. Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 Chapter 24 Chapter 25 Chapter 26
The Atomic Theory of Matter Dalton’s Atomic Theory Postulates 10 2. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of different elements are different. Different elements have different atomic properties such as atomic mass.
44 Flame Emission - it measures the radiation emitted by the excited atoms that is related to concentration. Atomic Absorption - it measures the radiation absorbed by the unexcited atoms that are determined. Atomic absorption depends only upon the number of unexcited atoms, the absorption intensity is not directly affected by the
Atomic Structure Worksheet **Assume all are neutral atoms! Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number Protons Neutrons Electrons Mass number
DEDICATION PART ONE Chapter 1 Chapter 2 Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 Chapter 9 Chapter 10 Chapter 11 PART TWO Chapter 12 Chapter 13 Chapter 14 Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 Chapter 20 Chapter 21 Chapter 22 Chapter 23 .