United States Environmental Protection Agency Office Of .
United StatesEnvironmental Protection AgencyOffice of Prevention, Pesticides and Toxic Substances(7505P)PesticideFact SheetName of Chemical:Reason for ionApril 2008Date Issued:TABLE OF CONTENTS1. Description of the Chemical .12. Use patterns and Formulations .23. Science Findings 34. Human Health Exposure Assessment 95. Environmental Exposure and Risk .116. Regulatory Position and Rationale .297. Reduced Risk Classification . .308. Contact Person .319. Appendix I: Glossary of Terms and Acronyms .3210. Appendix II: Bibliography .341. DESCRIPTION OF CHEMICALChemical 5-carboxamideEmpirical FormulaC18H14N5O2BrCl2Common Name:ChlorantraniliproleExperimental Name:DPX-E2Y45EPA PC Code:0901001
Chemical Class:Mode of Action:Anthranilic diamide insecticideInterruption of normal muscle contractionPesticide Type:InsecticideU.S. Technical Registrant:DuPont Crop ProtectionP.O. Box 30Newark, DE 19714-0030Chemical Structure:HNClONHONBrN NCl2. USE PATTERNS AND FORMULATIONSRegistered Uses:pome fruit (crop group 11), stone fruit (cropgroup 12), leafy vegetables (crop group 4),Brassica leafy vegetables (crop group 5),cucurbit vegetables (crop group 9), fruitingvegetables (crop group 8), cotton, grapes,potatoes, rice, and ornamentals and turfgrass growing in residential, commercial,and public landscaped areasPests/Application Sites:moths, beetles, caterpillars, etc.Application Rates:Seasonal Maximum:Food Crops- 0.2 lb a.i./acre(rice- 0.13 a.i./acre/year)Turf Grass- 0.5 lb a.i./acreOrnamentals- highly variable, rangebetween 0.33 to 0.5 lba.i./acreTypes of Formulations/Product Names:Technical:DuPont Rynaxypyr Technical (95.3% a.i.)End Use (Agricultural Uses):DuPont Coragen(18.4% a.i.; suspension concentrate)2
DuPont Altacor(35% a.i.; water dispersible granule)End Use (Turf and Ornamental Uses):DuPont E2Y45 SC Insecticide(18.4% a.i.; suspension concentrate)DuPont E2Y45 0.33G Insecticide(0.33% a.i.; granular)DuPont E2Y45 0.16G Insecticide(0.16% a.i.; granular)DuPont E2Y45 0.133G Insecticide Fertilizer(0.133% a.i.; granular)Manufacturing Concentrate (35% a.i.)3. SCIENCE FINDINGSPhysical and Chemical Characteristics:Available product chemistry data supporting the use of chlorantraniliprole aresummarized below in Tables 1 and 1.1.Table 1. Chlorantraniliprole Nomenclature.Chemical structureHNClONHONBrN NClCommon nameCompany experimental nameIUPAC nameCAS nameCAS registry mide500008-45-7Table 1.1. Physiochemical Properties of the Technical Grade TestCompoundParameterMelting point/range ( C)pHRelative DensityValue200-202 (95.9%)/208 – 210 (99.2%)5.77 0.087 at 20 C1.5189 (95.9%)/1.507 (99.2%) at 20 C3
Table 1.1. Physiochemical Properties of the Technical Grade TestCompoundParameterWater solubility (20 C)Solvent solubility (20 C)Vapor pressureDissociation constant, pKaOctanol/water partitioncoefficient, KOW (20 C)UV/visible absorption (max)ValueDeionized Water1.023 mg/LpH 40.972 mg/LpH 70.880 mg/LpH 90.971 mg/LAcetone3.446 0.172 g/LAcetonitrile0.711 0.072 g/LEthyl Acetate1.144 0.046 g/LDichloromethane2.476 0.058 g/LDimethylformamide124 4 g/Ln-Octanol0.386 0.01 g/LMethanol1.714 0.057 g/Ln-Hexane 0.0001 g/Lo-Xylene0.162 0.01 g/L6.3 x 10-12 Pa @ 20 C, 2.1 x 10-11 Pa @ 25 C10.88 0.71Deionized Water589pH 4588pH 7721pH 9654pH 2 no absorption max 200 nm, at 290 ε 3941pH 7 no absorption max 200 nm, at 290 ε 4185pH 10 absorption max at 320 nm which may bedue to decomposition of DPX-E2Y45, at 290 ε 6082Metabolism Assessment:The nature of the residue in plants and livestock is adequately understood. Verylittle degradation was observed in primary and rotational crops. Unchanged parentchlorantraniliprole was the major identified residue in primary and rotational crops. Themetabolism of chlorantraniliprole in livestock was extensive and followed the major stepssimilar to those observed in rice: (i) hydroxylation of the N-methyl group (to IN-H2H20)or hydroxylation of the tolyl methyl group (to IN-HXH44); (ii) cyclization with loss ofwater to a quinazolinone derivative (IN-EQW78); and (iii) N-demethylation via INH2H20 to IN-F9N04.Hazard Characterization:Toxicology RequirementsThe toxicology requirements (40 CFR 158.340) for a food use forchlorantraniliprole are in Table 2.4
Table 2. Toxicology Data 870.1200870.1300870.2400870.2500870.2600Acute Oral Toxicity.Acute Dermal Toxicity .Acute Inhalation Toxicity.Primary Eye Irritation.Primary Dermal Irritation .Dermal Sensitization 150870.3200870.3250870.3465Oral Subchronic (rodent).Oral Subchronic (nonrodent) .21-Day Dermal .90-Day Dermal .90-Day Inhalation.yesyesyesnonoyesyesyes-870.3700a Developmental Toxicity (rodent).870.3700b Developmental Toxicity (nonrodent) .870.3800 Reproduction 4200b870.4300Chronic Toxicity (rodent) .Chronic Toxicity (nonrodent).Oncogenicity (rat).Oncogenicity (mouse) es870.5100 Mutagenicity—Gene Mutation - bacterial.870.5300 Mutagenicity—Gene Mutation - mammalian.870.5385 Mutagenicity—Structural Chromosomal Aberrations.870.5395 e Delayed Neurotox. (hen) .90-Day Neurotoxicity (hen) .Acute Neurotox. Screening Battery (rat) .90-Day Neuro. Screening Battery (rat).Develop. Neuro .nonoyesyesnoyesyes-870.7485 General Metabolism .870.7600 Dermal Penetration.yesnoyes-Special Studies28-day immunotoxicity (rat) .28-day immunotoxicity (mouse).yesyesAcute ToxicityChlorantraniliprole Technical is toxicity category IV for all routes of exposureand is a non-sensitizer (Table 3).Table 3. Acute Toxicity of Technical .1300Study TypeMRID No.ResultsAcute oral toxicityAcute dermal toxicityAcute inhalationtoxicity468891124688911346889121LD50 5000 mg/kg bwLD50 5000 mg/kg bwLC50 5.1 mg/LToxicityCategoryIVIVIV5
870.2400Acute eye irritation46889115870.2500Primary skin irritation46889114870.2600Dermal sensitization46889221Iritis score of 1 in 1/3 rabbits, conjuctivalredness score of 1 in 2/3 rabbits. All eyesreturned to normal after 72 hours.No dermal irritation, clinical signs or bodyweight lossNot a dermal sensitizerIVIVNegativeSubchronic, Chronic and Other ToxicityIn short-term studies, the most consistent effects are those associated with nonadverse pharmacological response to the xenobiotic, induction of liver enzymes andsubsequent increase in liver weights. Chlorantraniliprole is not genotoxic, neurotoxic,immunotoxic, carcinogenic, or teratogenic. Overall, chlorantraniliprole exhibits minimalmammalian toxicity after long-term exposure. The only consistent observation in themammalian toxicology studies is an increased degree of microvesiculation of the adrenalcortex after dermal or dietary administration of chlorantraniliprole. Based on the lack ofadverse effect on the function of the adrenal gland, this observation was consideredtreatment related, but not “adverse.”Table 4. Subchronic, Chronic and Other Toxicity y)LOAEL(mg/kg/day)14-day OralGavage/ rat0, 25, 100, 10001000Notestablished28-Day Oral(feed)/rat0, 20.7, 106 and584 (male); 0, 24,128 and 675(female)584 (male)and 675(female)Notestablished28-Day Oral(feed)/mouse0, 52, 182, 538 and1443 (male); 0, 64,206, 658 and 1524(female)1443 (male)and 1524(female)Notestablished28-day Oral(capsule)/Dog0, 300, 10001000Notestablished28-day Oral(feed)/dog –Palatabilitystudy0, 26, 138, 266,797 and 1302(male); 0, 28, 138,298, 888, and 1240(female)0, 100, 300 and1302 (male)and 1240(female)Notestablished1000Not28-dayEFFECTSNo adverse effects. Weak inducer of cytochromeP450 3A at all dose levels, with statisticalsignificance at 100 and 1000 mg/kg/day.No adverse effects. Slight increase in liver weightat 128 and 675 mg/kg/day in females and minimalhepatocellular hypertrophy at 675 mg/kg that isattributed to enzyme induction characterized byincreased amount of eosinophilic cytoplasm withhepatocytes but no histomorphologic evidence ofhepatocellular damage. In 128 and 675 mg/kgfemales, a statistically significant increase in UDPGT activity was observed in HDT female rats, witha similar increase in males. These changes areconsistent with a pharmacological response andwere not considered adverse.No adverse effects. Slight increase in liver wt. in658 and 1524 mg/kg/day females correspondedwith a mild increase in cytochrome P450 enzymeactivity. No histopathological evidence of livertoxicity was observed.A reduction in body weight gain was observed inHDT males (52%) but not in females. Nostatistically significant decrease in absolute bodyweight was observed therefore, this effect was notconsidered adverse.No adverse effects. Induction of cytochrome P450enzyme activity (58%) in both males and females at1000 mg/kg/day, specifically 1A1 and 2B1/2 at 300and 1000 mg/kg/day.No adverse effects. Food consumption generallyincreased as the study progressed with malesgenerally demonstrating the highest foodconsumption when fed the HDT.No adverse effects. Reductions in mean body6
Table 4. Subchronic, Chronic and Other Toxicity L(mg/kg/day)1000LOAEL(mg/kg/day)established90-day Oral(feed)/rat0, 36.9, 120, 359,1188 (male); 0, 47,157, 460, 1526(female)1188 (male)and 1526(female)Notestablished90-day Oral(feed)/mouse0, 32.6, 115, 345,1135 (male); 0,40.7, 158, 422,1529 (female)1135 (male)and 1529(female)Notestablished90-day Oral(feed)/dog52-weekOral(feed)/dog0, 32.2, 119, 303,1163 (male); 0,36.5, 133, 318,1220 (female)0, 32, 112, 317,1164 (male); 0, 34,113, 278, 1233(female)1163 (male)and 1220(female)Notestablished1164 (male)and 1233(female)NotestablishedEFFECTSweight gain (22% and 19% for males and females)and food efficiency (19% and 17% for males andfemales) over the 28-day at the HDT.Increased microvesiculation of adrenal cortex inmales only, with no light or electronic microscopicevidence of adrenal cellular degeneration ortoxicity. No effect on the capacity of the adrenalgland to produce corticosterone under either basalor following ACTH stimulation. Therefore, theseeffects were not considered adverse.No adverse effects. A slight increase in liverweight at HDT females and reduction in bilirubinin females at 157 mg/kg/day, with nocorresponding histopathological evidence of livertoxicity.No adverse effects. Hyperactivity andhyperreactivity in females were observed near theend of the study and one male in the upper middose had convulsions, but these effects wereconsidered spurious as they were not reproduciblein the 18-month mouse study with a FOB.A slight increase in liver weight at the HDT malesand females, with no correspondinghistopathological evidence of liver toxicity.No adverse effects. A mild increase in liver weightwas observed in males at 1163 mg/kg/day, with nocorresponding histopathological evidence of livertoxicity.No adverse effects. A mild increase in liver weightin HDT males and females, and increase in alkalinephosphatase in HDT males, with no correspondinghistopathological evidence of liver toxicity.Body weight gain increase in HDT males for weeks8-9 compared to controls, with an increase in foodefficiency in week 9.No evidence of carcinogenicity and no adversefindings. Increased adrenal corticalmicrovesiculation due to lipid was present in thezona fasciculata region of the adrenal gland ofsome male rats in all dose groups in both the oneyear and main studies. This finding was consideredtest substance related but was not consideredadverse as the adrenal morphology was generally inthe range of what was observed in control rats, andthe finding was not associated with any indicationof cytotoxicity or other evidence of structural orfunctional impairment of the adrenal gland.2-Year Oral(feeding)/rat0, 7.71, 39, 156,805 (male); 0,10.9, 51, 212, 1076(female)805 (male)and use0, 2.6, 9.2, 26.1,158, 935 (male); 0,3.34, 11.6, 32.9,196, 1155 (female)158 (male)and 1155(female)935 (male),no LOAELestablishedfor femaleNo evidence of carcinogenicity. Eosinophilic fociaccompanied by hepatocellular hypertrophy andincreased liver weight form the bases for the maleLOAEL of 935 mg/kg/day.Twogenerationoralstudy/rat0, 200, 1000,4000, 20000ppm,mg/kg bw/d1199 (male)and 1594(female)NotestablishedA slight increase in mean liver weights in P1and F1 males and females at 238/318.9mg/kg/day and above, slight increase in meanadrenal weight at 238/318.9 mg/kg/day and7
Table 4. Subchronic, Chronic and Other Toxicity entalstudy/rabbitAcute useDOSES(mg/kg/day)equivalents:pre-mating:P1 m: 0, 12, 60,238, 1199F1 m: 0, 18, 89,370, 1926P1 f: 0, 16, 78,318, 1594F1 f: 0, 20, 104,406, 2178gestation:P1 f: 0, 14, 68,278, 1373F1 f: 0, 14, 71,272, 1465lactation:P1 f: 0, 32, 162,654, 3118F1 f: 0, 35, 183,696, 36410, 20, 100, 9/1594 mg/kg/day P1 and F1 males andfemales. Mean body weight of 1199/1594mg/kg/day F1 pups was slightly reduced onlactation days 7, 14 and 21. No effects on F2offspring weights during lactation.Minimal to mild increase in adrenal corticalmicrovesiculation in P1 adult males and F1adult males and females. P1 adult at 60.4/77.8mg/kg/day and greater. F1 adult males at 12mg/kg/day and greater. These effects were notobserved in weanlings. No cytotoxicity orabnormal cellular structures were observedunder light or electron microscopy.1000NotestablishedNo adverse effects.0, 20, 100, 300,10001000NotestablishedNo adverse effects.0, 200, 700, 2000in 0.5% methylcellulose0, 12.7, 64.2,255, 1313(male); 0, 15.1,77.3, 304, 1586(female)0, 74, 363, 1494(male); 0, 82,397, 1601(female)0, 48, 264, 1144(male); 0, 64,362, 1566(female)2000NotestablishedNo evidence of neurotoxicity was observed atany dose1313 (male)and 1586(female)NotestablishedNo evidence of neurotoxicity was observed atany dose.1494 (male)and 1601(female)Notestablished1144 (male)and 1566(female)NotestablishedNo evidence of treatment-related effects onthe sheep red blood cells specific antibody(IgM) responses in either male or female ratsat any dietary concentration tested.No evidence of treatment-related effect on thesheep red blood cells specific antibody (IgM)responses in either male or female mice at anydietary concentration tested.Food Quality Protection Act (FQPA) Decisions:The Agency concluded that the toxicology database is adequate for Food QualityProtection Act (FQPA) purposes and that there are no concerns or residual uncertaintiesfor pre-/post-natal toxicity. Therefore, a FQPA factor of 1X was selected. That decisionwas based on the following findings:a. The toxicology database for chlorantraniliprole is complete for the purposes ofthis risk assessment and the characterization of potential pre- and postnatal8
risks to infants and children.b. No susceptibility was identified in the toxicological database, and there are noresidual uncertainties re: pre-and/or postnatal exposure.c. There are no treatment-related neurotoxic findings in the acute and subchronicoral neurotoxicity studies in rats.d. The exposure assessment is protective: the dietary food exposure assessmentutilizes tolerance level residues and 100% crop treated information for allcommodities; the drinking water assessment utilizes values generated bymodels and associated modeling parameters which are designed to provideconservative, health protective, high-end estimates of water concentrations.By using these screening-level exposure assessments, the chronic dietary(food and drinking water) risk is not underestimated.e. Although residential exposure is expected over the short- and possiblyintermediate-term (via the dermal and/or incidental oral route), there is nohazard expected via these routes/durations, and therefore no risk for thesescenarios.4. HUMAN HEALTH EXPOSURE AND RISK ASSESSMENTResidue Profile:Dietary Exposure and Risk:Because an endpoint attributable to a single dose was not identified, the dietaryexposure assessment considered only chronic exposure, since chlorantraniliprole wasdetermined to be toxic only via the chronic oral exposure duration.Chronic dietary risk assessments were conducted using the Dietary ExposureEvaluation Model (DEEM-FCID , Version 2.03) which uses food consumption datafrom the U.S. Department of Agriculture’s Continuing Surveys of Food Intakes byIndividuals (CSFII) from 1994-1996 and 1998. The chronic assessments assumed that100% of crops with requested uses of chlorantraniliprole are treated, and that all treatedcrops contain residues at tolerance level.These assumptions result in conservative, health-protective estimates of exposurewhich are well below the Agency’s level of concern (100% of the cPAD). The maximumestimate is less than 1% of the cPAD for all population subgroups. These analysesindicate that there are no dietary exposure considerations that would preclude registrationof chlorantraniliprole for the requested uses.A drinking water assessment for chlorantraniliprole, conducted based onPRZM/EXAMS (Pesticide Root Zone Model/Exposure Analysis Modeling System), wasused to calculate the surface water estimated drinking water concentrations (EDWCs) and9
the Screening Concentration in Ground Water (SCI-GROW) model was used to calculatethe groundwater EDWC. The EDWCs do not exceed the Agency’s level of concern.Table 5. Results of Chronic Dietary Exposure and Risk Estimates /dayChronic EstimatesChronic Estimates(Food only)(Food and Drinking Water)Exposure,Risk, % cPADExposure,Risk, % cPADmg/kg/daymg/kg/day1.58U.S. Population0.007679 10.007756 1All infants0.007856 10.008108 1Children 1-2 yrs0.0149690.014855 1 1Children 3-5 yrs0.012043 10.012150 1Children 6-12 yrs0.007999 10.008073 1Youth 13-19 yrs0.005850 10.005906 1Adults 20-49 yrs0.007082 10.007154 1Adults 50 yrs0.007613 10.007689 1Females 13-49 yrs0.007215 10.007286 1The population subgroup with the highest estimated exposure/risk is bolded.Residential Exposure Estimates:Although there are only two use sites (turfgrass and ornamental plants), asindicated on the 14 terrestrial non-food end use products, these use sites encompass amultitude of places that may be treated: home lawns, commercial lawns, industrialfacilities, residential dwellings, business and office complexes, shopping complexes,multi-family residential complexes, institutional buildings, airports, cemeteries, interiorplantscapes, ornamental gardens, parks, wildlife plantings, playgrounds, schools, daycarefacilities, golf courses, athletic fields, sod farms and other landscaped areas. Themultitude of use sites, in addition to the persistence of chlorantraniliprole, indicates thereis potential for short- and intermediate-term postapplication dermal (adults and children)and incidental oral (children only) exposure to chlorantraniliprole (inhalation exposure isnot expected due to low vapor pressure). However, due to the lack of toxicity over theacute, short- and intermediate-term via the oral and dermal routes – no risk is expectedfrom these exposures.Long-term (greater than 6 months) dermal exposure to turfgrass is not expectedbecause the use pattern suggests a seasonal window of application, and dislodgeablefoliar residue (DFR) data indicate a maximum half-life of only 30 days on foliage. Whilechlorantraniliprole’s persistence in soil (half-life up to 1130 days in dissipation studies onbareground plots) increases the possibility of long-term exposure for toddlers viaincidental ingestion, the daily quantity of soil a toddler would need to eat to reach thecPAD is not feasible (more than 4 lbs/day, even when accounting for accumulation).Due to the lack of toxicity resulting from chlorantraniliprole exposure (other thanchronic oral ingestion), spray drift is not expected to pose a risk to residents near sprayingoperations.10
Aggregate Risk:Although there is potential exposure to chlorantraniliprole from food, drinkingwater and residential use sites, the only identified hazard is via the oral route over achronic duration. Residential exposures are expected to occur over a short- orintermediate-term duration. Therefore, the aggregate risk assessment considers onlyexposures from food and drinking water consumed over a long-term duration (greaterthan 6 months of daily exposure). That decision was based on the following findings:a.Acute Risk. No acute risk is expected because no acute hazard,attributable to a single dose, was identified.b.Chronic Risk. Using exposure assumptions, we concluded thatexposure to chlorantraniliprole from food and water will utilize 1% ofthe cPAD for the population group children 1-2 years (the highestexposed subpopulation). Based on the use pattern, chronic residentialexposure to residues of chlorantraniliprole is not expected.c.Short-Term/Intermediate Risk. There is potential for short- andintermediate-term post-application dermal (adults and children) andincidental oral (children only) exposure to chlorantraniliprole.However, due to the lack of toxicity via dermal route, as well as thelack of toxicity over the acute, short- and intermediate-term via the oralroute – no risk is expected from these exposures. Inhalation exposureis not expected due to the low vapor pressure of chlorantraniliprole (soapplied/deposited residues are not expected to volatilize into the air).d.Aggregate Cancer Risk. Chlorantraniliprole has been classified as a“not likely human carcinogen.” It is not expected to pose a cancer riskto humans.e.Determination of Safety. Based on the risk assessments, we concludethat there is a reasonable certainty that no harm will result to thegeneral population, or to infants and children from aggregate exposureto chlorantraniliprole residues.Occupational Exposure:The chlorantraniliprole toxicology database indicates there is no systemic hazardassociated with short- and intermediate-term dermal and inhalation exposure, andtherefore, no occupational exposure and risk assessment was conducted.5. ENVIRONMENTAL EXPOSURE AND RISKEnvironmental Fate Characteristics:Chlorantraniliprole may be characterized as persistent and mobile in terrestrialand aquatic environments. Extended chlorantraniliprole use is expected to cause11
accumulation of residues in soil from year to year. Major routes of dissipation areexpected to be alkaline-catalyzed hydrolysis, photodegradation in water, leaching, andrunoff.Nine degradates/metabolites of the parent compound have been identified inenvironmental fate studies: IN-EQW78, IN-LBA22, IN-LBA24, IN-LBA23IN-ECD73, IN-F6L99, IN-EVK64, IN-F9N04, and IN-GAZ70 (see Table 7). Thegreatest percentage production of a degradate was for IN-LBA24, which was 90% ofapplied parent produced in the photolysis study at pH7. The risk assessment did notquantify the risks from these degradates because they were commonly of lower toxicpotency than the parent. For example IN-LBA24 is orders of magnitude less toxic thanthe parent pesticide. Coupling the observed lower toxic potency with the riskassessments exposure modeling assumptions of stability for the parent would suggest thatexcluding the degradates from quantitative risk estimation would not substantially affectthe conclusion of the risk assessment.Table 6. Laboratory Environmental Fate Data forChlorantraniliproleDataUnitsValueMolecular WeightSolubilityg/molemg/L483.151.023Vapor PressureTorr1.57E-13Henrys ConstantHydrolysis @ pH 7Photodegradation in WaterAerobic Soil Metabolismatm m3/molDaysDaysDaysAerobic Aquatic MetabolismDaysAnaerobic AquaticMetabolismSoil:Water Coefficients 10.1246.6228.0888.6924.1396.0231125208L/g153-loam sand509-silty clay loam272-sandy loam526-loamy sand180-loamTable 7. Identified uctIN-EQW78Maximum FormationPercentage(% of applied parent)86.7 @ pH 9Chemical Name(2-[3-Bromo-1-(3-chloro-2-12
Table 7. Identified Degrades/MetabolitesStudyPhotodegradationin WaterSoil MetabolismDegradationProductIN-EQW78ND @ pH 7 buffer solutionND @ natural water, sterileIN-LBA2252.1 @ pH 7 buffer solution3.4 @ natural water, sterileIN-LBA2490.2 @ pH 7 buffer solution89.3 @ natural water, sterileIN-LBA2340.8 @ pH 7 buffer solution51.4 @ natural water, sterileIN-F6L992.1 @ 250C incubation5.2 @ 350C incubation4.2 @ 490C incubationIN-EVK64ND @ 250C incubation1.7 @ 350C incubation5.3 @ 490C incubation9.5 @ 250C incubation33.3 @ 350C incubation71.6 @ 490C incubationIN-EQW78Water/SedimentMetabolismMaximum FormationPercentage(% of applied parent)IN-ECD734.9 @ 250C incubation8.2 @ 350C incubation9.1 @ 490C incubationINGAZ704.3 @ 250C incubation7.4 @ 350C incubation1.0 @ 490C incubationIN-EQW7830.2 @ no photodegradation40.9 @ photodegradationIN-F6L994.2 @ no photodegradationND @ photodegradation2.7 @ no photodegradationND @ photodegradationIN-F9N04IN-GAZ703.0 @ no photodegradationND @ photodegradationIN-ECD734.7 @ no photodegradation0.8 @ photodegradationChemical Namepyridinyl)-1H-pyrazol-5-yl]-6chloro-3,8 -2pyridinyl)-1H-pyrazol-5-yl]-6chloro-3,8 azol-5-yl]carboxylic -5-yl]-6chloro-3,8 ol-5-yl]-6chloro-3,8 -5-yl]-6-13
Table 7. Identified Degrades/MetabolitesStudyDegradationProductMaximum FormationPercentage(% of applied parent)Chemical NLBA24INLNA2311.1 @ no photodegradationND @ photodegradation4.6 @ no photodegradation1.5 @ photodegradation2.3 @ no photodegradation0.5 @ photodegradationEcological Effects and Risk:Chlorantraniliprole can be characterized as having very little toxicity to terrestrialand aquatic vertebrates. As can be expected for an insecticide, the compound is toxic to anumber of terrestrial and aquatic invertebrates. The compound can produce limitedadverse effects in terrestrial and aquatic plants.Available data for formulated products suggested no concern for enhancedtoxicity of formulations versus the active ingredient alone. Data for degradates suggestno concern for toxicity exceeding the parent compound and in most cases toxicity isorders of magnitude below the parent.Terrestrial HazardBirdsChlorantraniliprole, degradates and formulated products can be characterized asbeing practically non-toxic from the acute oral and dietary perspectives. The availabledata show no indications that formulated product, metabolites, or degradates are moretoxic than the active ingredient.Table 8. Available Bird Toxicity Data for Chlorantraniliprole, Formulations, andDegradatesTest Nature ofTestedMaterialRegistrantStudy nicalDuPont14379Test usvirginianus(Northernbobwhite)Test TypeSubacutedietaryEndpoint TypeEffectsValueBasedon A.S.Units ofActiveSubstanceLC 50NOAECLOAEC (viableembryoreduction
UV/visible absorption (max) pH 2 no absorption max 200 nm, at 290 ε 3941 pH 7 no absorption max 200 nm, at 290 ε 4185 pH 10 absorption max at 320 nm which may be due to decomposition of DPX-E2Y45, at 290 ε 6082 Metabolism Assessment: The nature of the resid
INDICATORS OF FAECAL POLLUTION Valerie Harwood University of South Florida Tampa, United States Orin Shanks United States Environmental Protection Agency Cincinnati, United States Asja Korajkic United States Environmental Protection Agency Cincinnati, United States Matthew Verbyla San Diego State University San Diego, United States Warish Ahmed
PRECEDENTIAL SIERRA CLUB, Petitioner v. UNITED STATES ENVIRONMENTAL PROTECTION AGENCY *PENNSYLVANIA DEPARTMENT OF ENVIRONMENTAL PROTECTION, Intervenor Respondent *(Pursuant to the Court Order dated 8/5/19) _ On Petition for Review of Final Agency Action of the United States Environmental Protection Agency (EPA-1: EPA-R03-OAR-2017-0290)
PACIFIC COAST HIGHWAY P.8 United States THE ETERNAL WEST P.14 United States ROUTE 66 P.22 United States THE BLUES HIGHWAY P.24 United States THE KEYS: FLORIDA FROM ISLAND TO ISLAND P.26 United States ROUTE 550: THE MILLION DOLLAR HIGHWAY P.34 United States HAWAII: THE ROAD TO HANA P.42 United States OTHER
Index to Indiana Statistics in the Decennial Censuses Contents 3rd Census of the United States (1810) 2 4th Census of the United States (1820) 3 5th Census of the United States (1830) 4 6th Census of the United States (1840) 5 7th Census of the United States (1850) 7 8th Census of the United States (1860) 10 9th Census of the United States (1870) 17
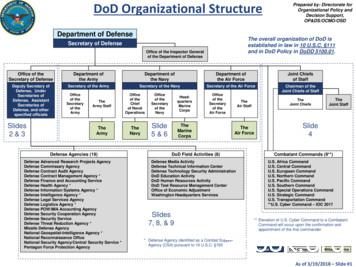
Defense Advanced Research Projects Agency. Defense Commissary Agency. Defense Contract Audit Agency. Defense Contract Management Agency * Defense Finance and Accounting Service. Defense Health Agency * Defense Information Systems Agency * Defense Intelligence Agency * Defense Legal Services Agency. Defense Logistics Agency * Defense POW/MIA .
U.S. Environmental Protection Agency i Notice Preparation of this commercialization assistance guide has been funded by the United States Environmental Protection Agen
Henry Spinelli, MD – United States Sherard A. Tatum, MD – United States Jesse A. Taylor, MD – United States Mark M. Urata, MD – United States John van Aalst, MD – United States Steven Wall, MD – United Kingdom S. Anthony Wolfe, MD – United States Vincent Yeow, MD – Singapore
Environmental Protection Agency Establishment The Environmental Protection Agency Act, 1992, was enacted on 23 Ap