CAP Accreditation And Checklists Update
CAP Accreditation andChecklists UpdateLyn Wielgos, MT(ASCP)Checklist Editor,CAP Accreditation ProgramsNovember 3, 2017
Objectives Discuss CAP Checklists and highlight changes in the 2017checklist edition Describe key changes for laboratories with Californiaclinical laboratory licensure Review tips for staying current with checklist changes Provide an update on implementation of individualizedquality control plan (IQCP) requirements 2017 College of American Pathologists. All rights reserved.2
What are the CAP Checklists? Detailed standards developed based on broad principlesdefined in the CAP Standards for Laboratory Accreditation– 21 different checklists with about 2,900 requirements Tool for laboratories to prepare for inspection Roadmap guide for inspectors to perform aninspection Customizable based on tests and activitiesperformed by the laboratory Updated annually based on input fromexperts in the field 2017 College of American Pathologists. All rights reserved.3
Summary of Changes in 2017 2017 College of American Pathologists. All rights reserved.4
Major Topics for the 2017 Update Laboratory General PersonnelSpecimen Collection and HandlingLaboratory Computer ServicesSafety/Physical FacilitiesLaboratories with California Licensure All Common Proficiency TestingInstruments & EquipmentTest Method Validation/VerificationIndividualized Quality Control Plans Director Assessment Checklist Discipline Specific Checklist Changes 2017 College of American Pathologists. All rights reserved.5
Laboratory General Checklist Changes 2017 College of American Pathologists. All rights reserved.6
Personnel: QualificationsGEN.54400 Personnel Records:Personnel records are maintained (in electronic or paper format)and readily available for all testing personnel, supervisorypersonnel, and other laboratory personnel Personnel trained outside of the US must have an equivalencyevaluation performed by a nationally recognized organization*such as:o National Association Credential Evaluation Services, Inc. (NACES)(http://www.naces.org)o Association of International Credential Evaluators, Inc. (AICE)(http://www.aice-eval.org)* A California laboratory personnel license or license to practice medicinein the state are acceptable evidence of equivalency assessment 2017 College of American Pathologists. All rights reserved.7
Personnel: TrainingGEN.55450 Personnel Training:There are records that all laboratory personnel havesatisfactorily completed training on all tasks performed, as wellas instruments/methods applicable to their designated job. Requires training for all laboratory personnel for all tasksperformed (including instruments) Clarifies that training must be completed prior to startingpatient testing Allows ongoing competency assessment records to be usedin lieu of training after the initial two year period (or five yearsfor transfusion medicine) 2017 College of American Pathologists. All rights reserved.8
Personnel: Competency AssessmentGEN.55500 The competency of each person performing patienttesting to perform his/her assigned duties is assessed Split into three separate requirements:o GEN.55499 Waived Testingo GEN.55500 Nonwaived Testingo GEN.55510 Qualifications to Assess CompetencyPoint-of-Care Testing Checklist revised to be consistent: POC.06875 Waived Testing POC.06910 Nonwaived testing POC.06920 Qualifications to Assess Competency POC.09600 Provider-Performed Microscopy (Nonwaived) 2017 College of American Pathologists. All rights reserved.9
Personnel: Competency Assessment, cont’dGEN.55500 Nonwaived Testing: Competency must be assessed at the specific laboratory(CAP/CLIA number) where testing is performed. All test performance variations must be included in thecompetency assessment specific to the site or laboratory. Records may be maintained centrally within a health caresystem. Records must be available upon request. 2017 College of American Pathologists. All rights reserved.10
Personnel: Competency Assessment, cont’dGEN.55499 Waived Testing: Laboratory director may determine how competency will beassessed for personnel performing testing at multiple testsites (same or different CAP/CLIA number). Variations in test performance at different test sites orlaboratories must be included in competency assessmentspecific to site or laboratory. Laboratories may continue to select which competencyassessment elements to assess. More stringent state or local regulations must be followed. 2017 College of American Pathologists. All rights reserved.11
Personnel: Competency Assessment, cont’dGEN.55510 Qualifications to Assess Competency: Assessor qualifications vary depending on the complexity oftesting:o High complexity - Section director/technical supervisor orindividual meeting general supervisor requirementso Moderate complexity - Technical consultant or individualmeeting those qualificationso Waived testing - Determined by the laboratory director 2017 College of American Pathologists. All rights reserved.12
Personnel: Supervision Deleted requirements in discipline specific checklists for“person in charge of bench testing” (eg, CHM.25800,HEM.40000):The person in charge of bench testing/section supervisor in chemistry haseducation equivalent to an associate’s degree (or beyond) in chemical, physicalor biological science or medical technology and at least four years ofexperience (one of which must be in clinical chemistry) under a qualified sectiondirector. Use supervision requirements in Laboratory General insteado GEN.53600 (General Supervisor) for high complexity testingo GEN.53625 (Technical Consultant) for moderate complexitytesting Change is consistent with CLIA roles and the CAP’sLaboratory Personnel Evaluation Roster 2017 College of American Pathologists. All rights reserved.13
Personnel: Supervision, cont’dGEN.53600 General Supervisor QualificationsSupervisors/general supervisors meet defined qualificationsand fulfill expected responsibilities. Revised NOTE: The general supervisor's training andexperience must be in the designated discipline or area ofservice for which the individual is responsible. Previous version only required training and/or experience inhigh complexity testing 2017 College of American Pathologists. All rights reserved.14
Specimen Collection and Handling:Chain of Custody Added six new requirements to the Laboratory GeneralChecklist. GEN.40502 Chain-of-Custody ProceduresGEN.40503 Chain-of-Custody RecordsGEN.40504 Chain-of-Custody Acceptability CriteriaGEN.40506 Secured Specimen StorageGEN.40507 Specimen Retention and StorageGEN.40509 Secured Records Removed “legal testing” section from the Chemistry &Toxicology Checklist Applies to:o Any collection process that follows a chain-of-custody (CoC)procedureo CoC testing referred to another laboratory Does not apply to laboratories in the RLAP or FDT programs 2017 College of American Pathologists. All rights reserved.15
Laboratory Computer ServicesGEN.43150 User AuthenticationThere are explicit written policies that specify who may accessthe computer system, how the access is obtained, and how thesecurity of access is maintained (e.g. inactivated whenpersonnel leave, not posted on terminals). Clarified to require written policies for:o Who may access the computer systemo How access is obtainedo How security of access is maintained 2017 College of American Pathologists. All rights reserved.16
Laboratory Computer Services, cont’dGEN.43200 User Authorization PrivilegesThere are written procedures and access privileges in place toconfine the level of access of authenticated users to thosefunctions they are authorized to use to fulfill their jobresponsibilities. Revised to:o Confine the level of access of authenticated users to thefunctions they are authorized to use 2017 College of American Pathologists. All rights reserved.17
Safety: Emergency PreparednessGEN.73800 Emergency PreparednessThere are written policies and procedures defining the role andresponsibilities of the laboratory in emergency preparednessfor harmful or destructive events or disasters. Previous version referred to internal and external disasters –now refers to harmful or destructive events or disasters. Revised NOTE to introduce a risk-based approach fordetermining the types of situations that must be addressedin the emergency preparedness plan. Based on a new CMS rule for Medicare and Medicaidproviders and suppliers. 2017 College of American Pathologists. All rights reserved.18
Safety: Emergency EyewashGEN.77400 Emergency Eyewash:The laboratory has adequate plumbed or self-containedemergency eyewash facilities . Focused requirement on potential exposure to the eye fromcorrosive chemicals (refer to SDS) May use a risk-based approach to determine appropriateplacement of eyewash facilities Clarified that availability of disposable eyewash bottles inwork area does not replace the need for an eyewash facility inareas at risk for eye exposure from corrosive chemicals 2017 College of American Pathologists. All rights reserved.19
Safety: VisitorsExpanded safety requirements to include provisions forlaboratory visitors: GEN.74000 Bloodborne Pathogens - include potentialhazards that visitors may encounter in exposure controlplan GEN.74100 PPE Provisions and Usage - make personalprotective equipment available to laboratory visitors 2017 College of American Pathologists. All rights reserved.20
Physical FacilitiesGEN.62020 Centralized Reagent and Supply StorageIf reagents and supplies are stored in a centralized area outsideof the laboratory, they are stored and handled in accordancewith the manufacturer's instructions, and temperatures arechecked and recorded daily using a calibrated thermometer. Added to address centralized storage areas outside of thelaboratory Requires storage and handling following manufacturer’sinstructions and daily temperature monitoring Storage of reagents and supplies in the testing areas willcontinue to be inspected with the All Common requirementCOM.30350 2017 College of American Pathologists. All rights reserved.21
Laboratories with California Licensure NEW section created in Laboratory General with 10requirements. Requirements included in customized checklists forlaboratories that have a California clinical laboratory license Applies to:o Most laboratories within the state of Californiao Other laboratories that test specimens that originate inCalifornia The CAP has been granted deeming authority with the stateto inspect for compliance with state law. 2017 College of American Pathologists. All rights reserved.22
Laboratories with California Licensure, cont’dRequirements focus on items unique to California law,including: Laboratory director and testing personnel qualificationsand licensure Qualifications and duties of unlicensed personnel andphlebotomists Posting of licenses and certificates Listing of the laboratory owner on the laboratory license Training program requirements Inclusion of the director’s name on patient reports Use of locked specimen storage boxes 2017 College of American Pathologists. All rights reserved.23
Laboratories with California Licensure, cont’dTwo cytopathology requirements were revised to addressdifferences for cytology workload recording (CYP.08500,CYP.08550): CYP.08500 - Manual screening of gynecologic smearslimited to 80 slides in a 24-hour period CYP.08550- Workload for cytotechs performing automatedand semiautomated gynecologic smears under Californiastate laboratory license limited to 200 gynecologic slidesin a 24-hour period 2017 College of American Pathologists. All rights reserved.24
All Common Checklist Changes 2017 College of American Pathologists. All rights reserved.25
Proficiency TestingCOM.01700 PT and Alternative Assessment Result EvaluationThere is ongoing evaluation of proficiency testing (PT) andalternative assessment results with appropriate correctiveaction taken for each unacceptable result.Revised NOTE:o Each unacceptable PT or alternative assessment result(any result or sample not meeting defined acceptabilitycriteria) must be evaluated. It is recommended that thelaboratory investigate acceptable results that showsignificant bias or trends. 2017 College of American Pathologists. All rights reserved.26
Proficiency Testing: InterlaboratoryCommunicationCOM.01800 PT Interlaboratory CommunicationThere is no interlaboratory communication about proficiencytesting samples until after the deadline for submission of data tothe proficiency testing provider.Revised NOTE for clarification: PT must be performed at the laboratory (CAP/CLIA number)for which it was ordered. PT must be reported at the laboratory where testing wasperformed. Laboratories sharing an LIS or personnel must follow strictpolicies and procedures to ensure that personnel do notaccess PT records from other laboratories. 2017 College of American Pathologists. All rights reserved.27
Instruments and Equipment: MicroscopesNEW microscope maintenance requirements added to the AllCommon Checklist (removed from discipline specificchecklists): COM.30680 Microscope Maintenanceo Properly maintained (cleaning, optically aligned)o Equipped for intended use (low, high dry, oil immersion lenses)o Preventative maintenance at least annually COM.30685 Microscopes for Fluorescence Testingo Monitoring for sufficient light sourceo Use of appropriate filters/slideso Minimization of ambient lighting during use 2017 College of American Pathologists. All rights reserved.28
Instruments and Equipment: PerformanceVerificationCOM.30550 Instrument/Equipment Performance VerificationThe performance of all instruments and equipment is verifiedprior to initial use, after major maintenance or service, and afterrelocation to ensure that they run according to expectations. Revised to further specify when/what must be verified. Clarified NOTE:o Instrument/equipment performance verification verification of test method performance specifications.o Instruments/equipment must perform according to expectationsfor intended use and within defined tolerance limits.o Appropriate function checks are required after relocation*.*This does not apply to portable equipment used following the manufacturer'sinstructions. 2017 College of American Pathologists. All rights reserved.29
Test Method Validation/VerificationMethod Performance Specifications (Nonwaived)Changes to the introduction: Added information to address the moving of instruments(FAQ):o If an instrument is moved, the laboratory isresponsible for determining that the methodperformance specifications are not affected by therelocation process or any changes due to the newenvironment (e.g. refer to the manufacturer's manualregarding critical requirements, such as set-uplimitations, environmental conditions, etc.). 2017 College of American Pathologists. All rights reserved.30
Test Method Validation/Verification, cont’dChanges to the introduction: Emergency Use Authorization (EUA):o Definition: The legal mechanism used by the FDA toallow the use of an unapproved medical product (eg,diagnostic device) or an unapproved use of an approvedmedical product during an emergency to diagnose, treat,or prevent a serious or life threatening disease conditioncaused by a chemical, biological, radiological, or nuclearagent (CBRN). 2017 College of American Pathologists. All rights reserved.31
Test Method Validation/Verification, cont’dEmergency Use Authorization (EUA) Often unable to validate (accuracy, precision, etc.) Must follow assay or test protocol without modification Document alternative assessment used to ensureaccurate test results 2017 College of American Pathologists. All rights reserved.32
Individualized Quality Control Plan (IQCP)COM.50200 List of IQCP’sThe laboratory has identified all tests using an IQCP on theCAP's List of Individualized Quality Control Plans form. Previously required two separate CAP forms for IQCP’s:o Summary Formo List Form IQCP Summary form discontinued Updated IQCP List form to include new fields (test sites,number of devices in use, implementation/revision date) Revised list form available on CAP.org (IQCP Resourcespage) 2017 College of American Pathologists. All rights reserved.33
Individualized Quality Control Plans (IQCP),cont’dCOM.50300 Risk AssessmentThe IQCP for a test/device/instrument includes a riskassessment to evaluate potential sources of error to include allof the following Clarified that in-house data collected for the risk assessmentmust support the frequency selected for external qualitycontrol (maximum interval between runs of external controls) 2017 College of American Pathologists. All rights reserved.34
Individualized Quality Control Plans (IQCP),cont’dCOM.50500 Quality Control Plan ElementsThe individualized quality control plan must define all aspectsmonitored based on the potential errors identified during therisk assessment, including the following parameters asapplicable:Modified NOTE: Removed provision that “external control material samplesmust be analyzed at least every 31 days” Laboratories may now define lesser frequency if supportedby the laboratory’s risk assessment and the manufacturer’sinstructions. 2017 College of American Pathologists. All rights reserved.35
Individualized Quality Control PlanCOM.50600 Quality Assurance MonitoringOngoing quality assessment monitoring is performed by thelaboratory to ensure that the quality control plan is effective inmitigating the identified risks for the IQCP and includesrecords of the following . Added a new statement based on CMS input:o “Reevaluation of the quality control plan if changes tothe reagents, environment, specimen, testing personnel,or test system elements of the risk assessment occur.” An example form for annual QA assessment of the IQCP isavailable on the CAP.org (IQCP Resources page). 2017 College of American Pathologists. All rights reserved.36
Director Assessment Checklist 2017 College of American Pathologists. All rights reserved.37
Director Assessment Checklist“Team Leader Assessment of Director & Quality Checklist”changed to “Director Assessment Checklist” to focus on role. “TLC” will continue to be used to identify eachrequirement (eg, TLC.10100, TLC.11425). All checklist versions generated on or after August 21,2017 will display the new name, including older editions(eg, 2015 and 2016). 2017 College of American Pathologists. All rights reserved.38
Director Involvement and Responsibilities Removed the “Director Not On-Site Full Time” section Revised existing requirements to strengthen and reinforcethe role of the director (whether routinely on-site or remote) Directors must perform an on-site assessment on a periodicbasis, which must be defined in a written policy (periodicityNOT defined by the CAP). 2017 College of American Pathologists. All rights reserved.39
Director InvolvementTLC.10435 Director InvolvementThe involvement of the laboratory director, including activitiesperformed on-site and through remote consultation isconsidered adequate by the laboratory administration, medicalstaff, and the inspection team, and follows written policy oragreement. 2017 College of American Pathologists. All rights reserved.40
Director Responsibilities: Self-InspectionTLC.10445 Director Responsibility – Interim Self-InspectionNEWThe laboratory director ensures that a thorough interim selfinspection is performed and all deficiencies are corrected in atimely manner. Focuses on director’s role to ensure that a thorough selfinspection is performed with correction of citeddeficiencies GEN.23584 Interim Self-Inspection – Revised to reinforcerecord retention, including records of corrective action 2017 College of American Pathologists. All rights reserved.41
Director Responsibilities: DelegationTLC.11425 Director Responsibility – Delegation of FunctionsRevised Must ensure that designees are qualified to performassigned duties and that duties are properly carried out May not delegate personal on-site assessment of physicaland environmental conditions and the adequacy of staffingto others - on-site assessment must be done on a periodicbasis (as defined in lab policy) Designees may not sub-delegate functions to others, exceptas in outlined in other requirements (see GEN.53400 andGEN.53600) 2017 College of American Pathologists. All rights reserved.42
Discipline Specific Checklist Changes 2017 College of American Pathologists. All rights reserved.43
Discipline Specific Checklist Changes, cont’d Anatomic Pathology – added NEW section Flow CytometryData Interpretation Cytopathology – added NEW section on ImmunochemistryStaining Microbiology – consolidated the Molecular Microbiologysection Chemistry and Toxicology – removed Legal Testing Chemistry and Toxicology/Molecular Pathology/ClinicalBiochemical Genetics – removed Radiation Safetyrequirements and consolidated them into Laboratory General 2017 College of American Pathologists. All rights reserved.44
Discipline Specific Checklist Changes, cont’d Point-of-Care Testing – renamed Provider-Performed Testingsection to Provider-Performed Microscopy and LimitedWaived Testing and updated terminology for consistency withCLIA Transfusion Medicine – updated requirements across thechecklist to clarify the intent and align with the FDA Histocompatibility – updated the Molecular HLA Testingsection Histocompatibility/Molecular Pathology – added a NEWsection Stem Cell Engraftment Monitoring 2017 College of American Pathologists. All rights reserved.45
How to Keep Up to Date 2017 College of American Pathologists. All rights reserved.46
Resources Available on CAP.org 2017 College of American Pathologists. All rights reserved.47
Checklist Download: e-Lab SolutionsChecklist Type Options: Master Custom Changes OnlyChecklist Format Options: PDF Word/XML Excel 2017 College of American Pathologists. All rights reserved.48
Top Ten Deficiencies: 2016 Inspection Data 2017 College of American Pathologists. All rights reserved.49
Individualized Quality Control Plan UpdateApproximately 1500 deficiencies were cited in 2016 related toIQCP (55% All Common & 44% discipline specific checklists) Majority of All Common checklist deficiencies due toincomplete IQCP––––Missing CAP list and summary formIncomplete risk assessmentMissing director signatureQuality assessment monitoring not defined 2017 College of American Pathologists. All rights reserved.50
IQCP - 2016 Deficiency DataThe following chart shows the distribution of deficienciesbased on the discipline-specific checklist used for inspection: 2017 College of American Pathologists. All rights reserved.51
IQCP – Most Common Discipline-SpecificDeficienciesMost common reasons why laboratories were cited: Laboratories misunderstood the complexity of testingperformedo Waived vs. nonwaivedo Use of different specimen type changed complexity Laboratories continued to follow Equivalent QualityControl (EQC) regulations and did not implement an IQCP IQCP implemented after the January 1, 2016 deadline Microbiology laboratories continued to follow CLSIguidelines for media, ID, and/or susceptibility withoutimplementing an IQCP 2017 College of American Pathologists. All rights reserved.52
SummarySuccess is not about your resources. It’s about howresourceful you are with what you have – TonyRobbins 2017 College of American Pathologists. All rights reserved.53
2017 Focus on Compliance Webinars 2017 College of American Pathologists. All rights reserved.55
ResourcesChecklist interpretation questions? Email: accred@cap.org Phone: 1-800-323-4040, option 1 2017 College of American Pathologists. All rights reserved.56
2017 College of American Pathologists. All rights reserved.57
Checklist. GEN.40502 Chain-of-Custody Procedures GEN.40503 Chain-of-Custody Records GEN.40504 Chain-of-Custody Acceptability Criteria GEN.40506 Secured Specimen Storage GEN.40507 Specimen Retention and Storage GEN.40509 Secured Records Removed “legal testing”
This pdf contains 77 electrical inspection checklists taken from the 2014 Electrical Inspection Manual with Checklists. The checklists are in PDF format and can be completed electronically or printed and used as hard copy. The checklists are intended to help inspectors keep track of the numerous aspects of an electrical installation
Practice Accreditation Program Web based program launched in January 2011 Application, interview and data collection forms, surveyor report and summary are all captured electronically No more paper ACR-ASTRO accreditation outcomes 3 Categories: Accreditation Defer Denial of Accreditation ACR-ASTRO Accreditation Accreditation Cycle is 3 years
Cap. 1 Fondamenti di informatica e hardware Cap. 2 Il software Cap. 3 La rappresentazione dei dati per le scienze umane Cap. 4 Dalle reti a Internet Cap. 5 Il World Wide Web Parte II - Temi per le scienze umane Cap. 6 La computazione linguistica Cap. 7 Arte e beni culturali nell'era digitale Cap. 8 Biblioteconomia e ricerca delle informazioni .
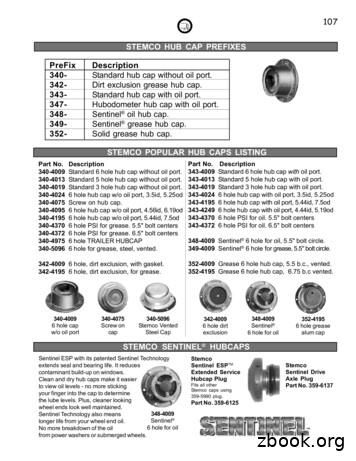
347-Hubodometer hub cap with oil port. 348-Sentinel oil hub cap. 349-Sentinel grease hub cap. 352-Solid grease hub cap. Part No. Description 340-4009 Standard 6 hole hub cap without oil port. 340-4013 Standard 5 hole hub cap without oil port. 340-4019 Standard 3 hole hub cap without oil
Callan Periodic Table of Investment Returns Returns Ranked in Order of Performance (as of June 30, 2019) Equity Cap Large-9.11% Equity Cap Large-11.89% Equity Cap Large-22.10% Equity Cap Large 28.68% Equity Cap Large 10.88% Equity Cap Large 4.91% Equity Cap Large 15.79% Equity Cap Large
FLIGHT PLAN CLOSE DA-40F CHECKLISTS DA-42 CHECKLISTS ARROW CHECKLISTS. 10 Rev. 11.17 DA-40F NORMAL CHECKLIST AirCrAFt seCUre Tie-downs SECURED Chocks INSTALLED (main ldg gear) Pitot Cover INSTALLED Gust Lock INSTALLED Stall Warning Cov
The Accreditation Criteria are divided into three levels. To achieve Provisional Accreditation, a two year term, providers must comply with Criteria 1, 2, 3, and 7–12. Providers seeking full Accreditation or reaccreditation for a four-year term must comply with Criteria 1–15. To achieve Accreditation with
Accreditation Application Review Process The accreditation application review is the first step in the accreditation process. Once submitted, the SSH Accreditation staff will review the application. If the application is complete and all eligibility criteria met, an on-site review will be scheduled. Accreditation On-Site Survey Process