Welcome To Our CHEM 4 Lecture Review Question: Session ID
Welcome to our CHEM 4 lectureReview question: Gas forming reactionsGo to LearningCatalytics.com Session ID 1) Which of these reactions is not expected to produce a gas as one of the products? [Forpractice also write the balanced molecular equation for each gas forming reaction.]A)B)C)D)E)Na2S(aq) HClO4(aq)NaOH(aq) NH4I(aq)NaHSO3(aq) HNO3(aq)HC2H3O2(aq) Na2CO3(aq)HBr(aq) Na2SO4(aq)A)B)C)D)E)sulfide acidbase ammoniumhydrogen sulfite acidacid carbonateacid sulfateHere are the balanced molecular equations for the 4 gas forming reaction:A)Na2S(aq) 2HClO4(aq) H2S(g) 2NaClO4(aq)B)NaOH(aq) NH4I(aq) NH3(g) H2O(l) NaI(aq)C)NaHSO3(aq) HNO3(aq) SO2(g) H2O(l) NaNO3(aq)D)2HC2H3O2(aq) Na2CO3(aq) CO2(g) H2O(l) 2NaC2H3O2(aq)1
Exam #3: Information Exam #3 is Friday, December 4. During normal class period. Go to Canvas to take the exam. Timed: 50 minutes 20 multiple choice questions; worth 5 pts each. Both questions and answers will be randomized for each student. Can use class handouts, textbook, lecture notes, PowerPoint slides. Get all your materials (such as handouts, calculator and paper/pencil) readybefore you start the exam. Even though it is open book, you will not have enough time to look up everysingle thing, so you must study and be fully prepared going into the exam.2
Exam #3: ResourcesOctober calendar: tinyurl.com/SacStateChem4 Learning Outcomes for Exam #3. PowerPoint slides and recordings of lecture. Practice exams, 4 versions: A, B, C, and D. [NOTE: they are not on Canvas] Time yourself; take it like a real exam. Make a list of the type of questions you are getting wrong and focus yourstudy on those topics. For extra practice on those topics: Video recording of lecture, PowerPointslides, e-text, optional homework problems, PAL worksheets. Finish up any late homework for credit.Need help? Jeff’s office hours this week: MW 9 – 9:30 am and 11 – 11:30 am. Review session, Wednesday (12/2) during lecture. New format: I’ll use clickersto have everyone vote on which questions from the first 2 practice exams (Aand B) they want me to go over. Be prepared to vote. PAL office hours: link is on our CHEM 4 website PAL study hall (open to all CHEM 4 students): TBD Zoom code:3
Academic dishonesty: Cannot use any online resources that are not explicitly associated with class. Students posting to sites like Chegg, Bartleby, or Study.com are cheating. Remember: Everyone get’s hurt by cheating: Cheaters are stealing the hard work of others by taking a grade that they haven’tearned. Cheaters hurt themselves because they won’t be prepared for our next exam orfor CHEM 1A/1E, not to mention the MCAT, EIT, DAT, PCAT. Cheaters risk getting caught and being brought up on disciplinary charges. SacState’s reputation is hurt when employers realize our grads don’t knowanything! Bottom line: There is no reason to cheat in this class. You are smart enough to earna good grade. So, do your studying and be proud of the grade that you earn. My promise to you: There will be no surprises and no trick questions. I just want tosee if you have been learning the material that we’ve covered.4
CHEM 4 lectureMonday – November 30, 2020Sec 7.9 – 7.10Types of reactions5
Reading question: Types of reactions (Sec 7.9-7.10)Go to LearningCatalytics.com Session ID 2) Which of the following statements about oxidation-reduction reactions isfalse?A) Oxidation-reduction reactions require oxygen as a reactant.B) Oxidation-reduction reactions are also called redox reactions.C) Reduction is the gain of electrons.D) Oxidation-reduction reactions involve the transfer of electrons fromone substance to another.E) If one substance is oxidized, then another substance must be reduced.F) Oxidation is the loss of electrons.G) Combustion reactions are a type of oxidation-reduction reaction.6
Remember LEO goes GERLose Electrons OxidizedGain Electron Reduced7
Background: Redox reactions Redox reactions are one of the most important categories of reactions: Combustion reactions: Fuel O2(g) CO2(g) H2O(g)8
Background: Redox reactions Electrochemical reactions and batteries9
Background: Redox reactions Cellular respiration and biological processes10
Background: Redox reactions Not all redox reactions are good: Rusting and corrosion11
Background: Identifying redox reactions Redox reactions involve something gaining electrons (reduction) and somethinglosing electrons (oxidation). Can’t have one without the other. Easiest way to tell is to look at the oxidation numbers, Ox # (not in our textbook). If the Ox # changes during the reaction, then it is a redox reaction. Rules for determining Ox # are complex, but for now only 2: Ox # for a pure element 0 (ex. Ox # for H2, Ne, Cu 0) Ox # for an ion ion charge (ex. Ox # for KCl 1 for K and -1 for Cl) Ex.4 Na (s) O2 (g) 2 Na2O (s)Ox # 00 1, -2each Na lost 1 e- (oxidized)each O gained 2 e- (reduced) To make it even easier for CHEM 4, on tests, you’ll always have a pure element (Ox# 0) on one side of the reaction that becomes part of a compound (Ox # 0).12
Clicker question: Identifying redox reactions3) Which of the following reactions is not a redox reaction?A) H2(g) S(s) H2S(g)B) C3H8(g) 5 O2(g) 3 CO2(g) 4 H2O(g)C) HCl(aq) NaOH(aq) H2O(l) NaCl(aq)D) H2(g) F2(g) 2 HF(g)In addition to being a redoxreaction, B) is also acombustion reaction:Fuel O2 (g) CO2(g) H2O(g)E) 2 Fe(s) 3 Cl2(g) 2 FeCl3(s)C) is an acid-base neutralization reactionand a double displacement reaction.13
Clicker question: Writing redox reactions4) Write the balanced equation for the combustion of octane, C8H18. What iscoefficient in front of the CO2?A) 8C) 18E) 16B) 4D) 6F) 2Answer:C8H18 (l) O2 (g) CO2 (g) H2O (g)2 C8H18(l) 25 O2(g) 16 CO2(g) 18 H2O(g)Combustion reaction fuel O2 CO2(g)H2O14
Big picture: Types of chemical reactionsBe able to: Classify any given reaction according to this scheme Predict products for any of the reactions (except redox) Precipitation, acid-base, and gas forming double displacement Combustion form CO2 and water Balance any of them15
Clicker question: Answer this question based onyour assigned reading (Sec 7.10) for today.5) Which of the following generic reactions is not correctly classified?A)B)C)D)E)AB CD AD CBAB A BA B ABA BC AC BA B Cis a double-displacement reactionis a decomposition reactionis a synthesis reactionis a displacement reactionis a conversion reaction16
Clicker question: Identifying types of reactions6) Which of these reactions is not correctly classified using the codes as hesisdecompositiondisplacementdouble displacement2 HNO3 (aq) Ca(OH)2 (aq) 2 H2O (l) Ca(NO3)2 (aq)2 HNO3 (aq) SrS (aq) H2S (g) Sr(NO3)2 (aq)N2(g) 3 H2(g) 2 NH3(g)Li2SO4 (aq) SrCl2 (aq) SrSO4 (s) 2 LiCl (aq)2 C8H18 (l) 25 O2 (g) 16 CO2 (g) 18 H2O (g)Fe(s) Ni(NO3)2(aq) Ni(s) Fe(NO3)2(aq)2, 93, 94, 61, 94, 51, 8F) is 4 and 817
A) Oxidation-reduction reactions require oxygen as a reactant. B) Oxidation-reduction reactions are also called redox reactions. C) Reduction is the gain of electrons. D) Oxidation-reduction reactions involve the transfer of electrons from one substance to another. E) If one substance is oxidized, then another substance must be reduced. F .
CHEM 350B Topics in Chemistry 7.5 454.95 CHEM 351 Chemicals Big and Small: Nano- 15 909.90 CHEM 352 Advanced Concepts in Chemistry 15 909.90 CHEM 352A Advanced Concepts in Chemistry 7.5 454.95 CHEM 352B Advanced Concepts in Chemistry 7.5 454.95 CHEM 360 Contemporary Green Chemistry 15 909.90 CHEM 380 Materials Chemistry 15 909.90
CHEM 31X. Chemical Principles 4 CHEM 33. Structure and Reactivity 4 CHEM 35. Organic Monofunctional Compounds 4 CHEM 36. Organic Chemistry Laboratory I 3 MATH 41, 42, 51. Calculus, Linear Equations 5 5 5 SECOND YEAR CHEM 130. Organic Chemistry Laboratory II 4 CHEM 131. Organic Polyfunctional Compounds y3 CHEM 134.
CHEM 110 Chemistry of the Living World 15 4,736.85 CHEM 120 Chemistry of Material World 15 4,736.85 CHEM 150 Concepts in Chemistry 15 4,736.85 CHEM 200 Special Topic 15 4,736.85 CHEM 251 Structure and Spectroscopy 15 4,736.85 CHEM 252 Properties and Analysis of Mat 15 4,736.85
WRF-Chem Version 3.9.1.1 User’s Guide Table of Contents 1.1 WRF-Chem Introduction3 1.2 WRF-Chem software 5 1.3 Possible applications of the current modeling system 5 1.4 The WRF-Chem modeling system overview 5 2.1 Software Installation Introduction 8 2.2 Building the WRF-Chem code 9 2.2.1 Getting the code 9
bonding and reactions) necessary for courses in elementary organic chemistry and physiological chemistry. Students may only receive credit toward graduation for one of the following: CHEM 10050; or CHEM 10060 and CHEM 10061; or CHEM 10970 and CHEM 10971.
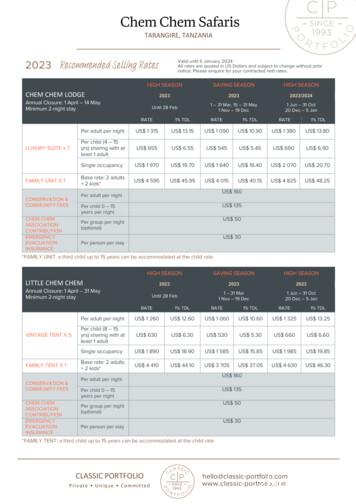
Chem Chem Safaris TARANGIRE, TANZANIA Updated: 9 November 2022 Page: 2 of 6 www.classic-portfolio.com CLASSIC PORTFOLIO Private Unique Committed hello@classic-portfolio.com www.classic-portfolio.com FOREST CHEM CHEM Annual Closure: 1 April - 31 May Minimum 2-night stay SAVING SEASON HIGH SEASON 2023 2023/2024 1 - 31 Mar 1 Nov - 19 Dec
1) Biology majors should take CHEM 104, CHEM 105 and CHEM 140 as early as possible in their B.Sc. program. (You should be taking CHEM 104 this semester, and should take at least one of CHEM 105/140 this coming January). 2) Students looking to take an elective Biology course are welcome in BIOL 100, but should
3. Most students are required to take Chem 103, General Chemistry Laboratory, concurrently with Chem 102. This 1 hour lab course provides active demonstration of principles covered in Chem 102B/C and introduces experimental skills. eference the R Chemistry 103 Experiments book for details concerning Chem 103.Chem 103 starts the week of .