Polymer Synthesis
Polymer Synthesis Final LabPolymer chemistry (Rayon Synthesis)Pages 317 - 330Pre-lab: Page 328 Post-Lab: Page 330Part of this experiment is NOT in yourmanual see additional handouts.
What is a polymer? Molecules substances of high molecularmass formed by joining together individualmolecules called monomers Polymers are either natural or synthetic.Natural polymers such as proteins, DNA,starch, and cellulose occur in nature.Synthetic polymers are chemicallyprepared.
The physical properties of polymers can varytremendously. For example, some polymers are extremelyflexible and fluid, whereas others are very hardand stress-resistant. One way to classify polymers is based ondifferences in elasticity. Elasticity refers to the ability of a polymer tostretch and return to its original shape. Elastomers are highly flexible and very elastic.One factor that affects the elasticity is the type ofmonomer group. Rubber, for example, is veryelastic and is made from isoprene monomers.
Rubber, for example, is very elastic and is madefrom isoprene monomers In contrast, polymeric fibers have a much lowerdegree of elasticity. The chains of a fiber are in a highly orderedarrangement, making it more difficult to stretch.Nylon, Dacron, and acrylics (Orlon) are all fibers. In between elastomers and fibers are plastics,which demonstrate intermediate characteristics.
Cross Linking Cross linking may increase the strength of apolymer. Cross linking is a type of bonding thatoccurs between two chains of polymer. As the amount of cross linking increases,the polymer become less flexible. Often, an agent is needed to promote crosslinking.
When you make slime you will use a crosslinking agent. The diagram below depicts cross linking The wavy lines between polymer 3 and 4represent cross linking bonds.
Polymers can also be formed by eliminationof a small molecule, often water This process is called condensation The product is called a copolymer if twodifferent molecules are involved in thepolymerization Nylon 6,6 is an example of a copolymer
O OH H HO C (CH 2 ) 4 C OH H N (CH 2 ) 4 N Hadipic acidO hexamethylene diamineO HH HO C (CH 2 ) 4 C N (CH 2 ) 4 N H H 2O Each end of the chain can undergoadditional condensation reactions The result is the silk substitute commonlycalled nylon
The characteristics of a polymer can be changeddramatically by connecting different polymerchains These connections or bridges are called crosslinks Perhaps the best know example of this isvulcanized rubber Vulcanization is the process of adding sulfur tonatural rubber latex and then heating
Natural rubber latex ismade much moreuseful by adding crosslinks. (a) Two strandsof polyisoprene(rubber). (b) Sulfurreacts by opening thedouble bonds in thepolymer and formingbridges betweenadjacent strands. Theproduct is calledvulcanized rubber.
Cross Linking Poly(vinyl alcohol) to formSlime In this lab experiment, you will synthesize Slimefrom poly(vinyl alcohol). Poly(vinyl alcohol) or PVA is a polymer with achemical formula (CH(OH)CH2CH(OH)CH2)n. PVA is used in many applications such as coatinggrease-proof paper, artificial sponges, andthickening food. However, PVA is not Slime! Slime is produced bycross-linking PVA polymers with borate anions,[B(OH)4-]. When borax is added to water, borateanions form.
The reaction that forms Slime is shown below.The oxygen atoms on the borate anion can interactwith the alcohol group (OH group) of PVA. This weak interaction is called hydrogenbonding and is also responsible for the 3dimensional structures of proteins. These weak interactions can break and reformcontinuously, if the correct amount of water ispresent. In the picture below, the dotted lines representweak hydrogen bonds forming an extended latticethroughout Slime.
Slime is very flexible when it containsenough water. When it is at rest, the Slime becomes rigid.Though each hydrogen bond alone is veryweak, all together the hydrogen bonds forma rigid structure. This network of hydrogen bonding is easilydisrupted by deformation through handling,squeezing, stirring, and pouring.
As Slime dries out, the amount of water decreasesand the slime becomes hard. If too much water is added, the weak hydrogenbonding linkages between -OH and borate becomeseparated so that the Slime no longer gels. This situation is not easily reversed until enoughwater has evaporated from the Slime.
Synthesizing a Silicate Chain to Formrubber balls In the second part of the lab experiment,you will synthesize an inorganic polymerbased on silicon. Inorganic polymers are polymers with anon-carbon backbone. Silicon is an element directly below carbon,yet the chemistry of carbon and silicon arevery different. Silicones form very stable polymers and areused in many products.
Heating dimethyldichlorosilane (Me2SiCl2)with water produces polymethylsilicone(Me2SiO)n, a polymer of high thermalstability. Adding methyltrichlorosilane (MeSiCl3) asa cross linking agent to polymethylsilicone(Me2SiO)n, forms a rubber "silly putty" or ahard resin, depending on the degree of crosslinking. Resins are commonly used to form plasticobjects like cups and bottles.
When sodium silicate solution (Na2Si3O7 in H2O)is added to ethyl alcohol, a polymer is formed. Sodium silicate solution contains sodiumhydroxide (NaOH) and silicon dioxide (SiO2). Sodium hydroxide is a strong base. Under thesebasic conditions, silicate chains form. When ethyl alcohol (CH3CH2OH) is added tosodium silicate solution, two oxygen atoms ofsilicate are replaced by ethyl (CH2CH2) with lossof water.
Overall reaction:
Cellulose Cellulose is a naturally occurring organicpolymer, found in plants and algae. Cellulose is the major constituent of paperand cardboard and of textiles made fromcotton, linen, and other plant fibers. It is derived from repeating units of glucose.
CelluloseCellulose is astraight chainpolymer: unlikestarch, no coilingoccurs, and themolecule adopts anextended and ratherstiff rod-likeconformation.
Cellulose is not very soluble in organicsolvents. This is because it has a very highmolecular weight. Tetraamminecopper(II) hydroxide dissolvescellulose giving smaller molecules that reactwith water (hydrolyse) and form a moresoluble species.
Rayon Synthesis Tetraammine copper(II) hydroxide will beprepared in a 2 step synthesis fromcopper(II) sulfate and ammomia, followedby removal of the sulfate ion, by addition ofBa2 and addition of a concentratedammonia solution. Reaction of the copper complex withcellulose helps break up the polymerthrough electrostatic interactions.
The rayon is precipitated through reactionof the basic solution with sulfuric acid. A pale blue color remains due to thecopper(II) ions that are on the surface. The color can be removed by washing thepolymer with DI water.
Note: Step 10, page 324, sulfuric acid will beprepared. Step 11, page ,325 the spinning apparatuswill not be prepared, alternative methodswill be used. Wear gloves to handle your rayon, it willstill have acidic residues present.
Inorganic polymers are polymers with a non-carbon backbone. Silicon is an element directly below carbon, yet the chemistry of carbon and silicon are very different. Silicones form very stable polymers and are used in many products.
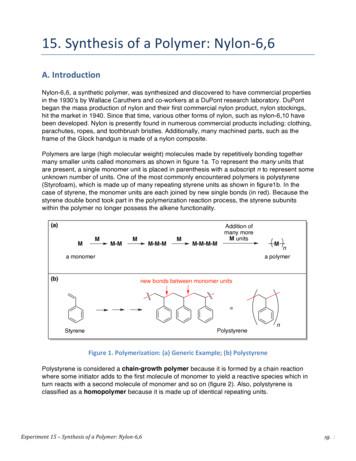
Experiment 15 – Synthesis of a Polymer: Nylon-6,6 pg. 1 15. Synthesis of a Polymer: Nylon-6,6 A. Introduction Nylon-6,6, a synthetic polymer, was synthesized and discovered to have commercial properties in the 1930’s by Wallace Caruthers and co-workers at a DuPont research laboratory. DuPont
Experiment 21 – Synthesis of a Polymer: Nylon-6,6 pg. 1 21. Synthesis of a Polymer: Nylon-6,6 A. Introduction Nylon-6,6, a synthetic polymer was synthesized and discovered to have commercial properties in the 1930’s by Wallace Caruthers and co-workers at a DuPont research laboratory. DuPont
Polymer Indexing Reference Manual – contains Polymer Indexing Code List, Polymer Indexing Molecular Formula List and Polymer Indexing Chemical Aspects Graphical Definitions. Polymer Indexing System Description – provides a detailed description of the Enhanced Polymer Index. CPI Plasdoc Coding Systems - provides details of the
as reinforcements for polymer composites. This replacement could be again synthetic, petroleum-based polymer but prepared as fibers, micro- or nanofibrils. Of course, this approach is not as advantageous as using natural fibers that are biodegradable and eco-friendly. At the same time, the synthetic polymer-polymer composites seem to be much
liquid (polymer melt) partially crystallised perfect crystal polymer Classification of states for polymer materials: 1. Partially crystalline state 2. Viscoelastic state (polymer melt) 3. Highly elastic state (e.g., rubbers) 4. Glassy state (e.g., organic glasses from poly(styrene), poly(methylmethacrylate), etc.) Polymer solutions.
Baeropol "One-Pack" products are used globally by polymer producers, polymer recyclers, compounders and polymer converters to protect their polymer products from degradation. Baeropol custom formulated blends can contain a variety of additives to protect polymer properties and enhance the polymer processing characteristics for speci c end use applications.
Important types of modified polymer systems include polymer composites, poly-mer–polymer blends, and polymeric foams. 1.3.1 Types and Components of Polymer Composites Polymer composites are mixtures of polymers with inorganic or organic additives having certain geometries (fibers, flakes, spheres, particulates). Thus, they consist of
Polymer Gels Boulder Lectures in Soft Matter Physics July 2012 M. Rubinstein and R.H. Colby, “Polymer Physics” (Oxford, 2003), Chapters 6 and 7 P. -G. de Gennes, “Scaling Concepts in Polymer Physics” (Cornell, 1979), Chapter 5 S. V. Panyukov and Y. Rabin, “Statistical Physics of Polymer Gels”, Phys. Rep.