Physics (Code-A) Solutions Of Sample Question Paper For .
Physics (Code-A)Solutions of Sample Question Paper for Class XIIPhysics (Theory) - Class XII (Code-A)SOLUTIONSSECTION-A1.No change, resistivity is material dependent.2.Electric and magnetic fields. Transverse waves.3.Diffraction of light occurs when size of obstacle (or aperture) is comparable to the wavelength of light used.4.By Lenz law it is in clockwise direction.5.When image is formed at distinct vision,M 1 D25 1 65fWhen image is formed at infinity,M 6.KEmax eVSP VSP7.D 25 55f3 eV 3 volt.eR - Remains unchangedXL - Becomes doubledXC - Becomes halved8.Since dipole consists of equal and opposite charge hence net charge and thus net flux is zero.SECTION-B9.80 F and 10 F are in parallelTherefore, equivalent is 80 10 90 FThis is in series with CHence, equivalent90C 4090 C C 72 F(1)
Solutions of Sample Question Paper for Class XIIPhysics (Code-A)10. (a) Outside the shell, qenc E ds 0Rq E 4 r 2 0 E r Rq4 r 2 0(b) Inside the shell, Take Gaussian surface qenc E ds r0as qenc 0R E 011. The property of an electric circuit by virtue of which it opposes any change of flux or current in it by inducing acurrent in itself is called self induction. It is numerically equal to the flux linked when unit current flows through it. i Li iSI unit is henry.L Consider, air cored solenoidII BAN 0 ni r 2 nl L 0 n 2 r 2 li12. Ampere’s circuital law : The magnetic circulation around any closed curve is equal to 0 times the electriccurrent threading the curve.Amperian curve 0Ienclosed B dl rMagnetic field due to a toroid B dl 0IenclosedB 2 r 0NIB 0NI2 r(2)Currentin eachturn is I
Physics (Code-A)Solutions of Sample Question Paper for Class XII13. Power of lens is a measure of its ability to converge or diverge a light beam falling on it. Power of lens is definedas the tangent of the angle by which the lens converges (or diverges) a beam of light falling on it at a unit distancefrom its optical centre.tan hfwhen h 1, tan 1fh1fPower of a lens P tan fUnit of power of lens is dioptre (D) or m–1.When lenses are in contactPeq P1 P214.Magnification :(a) For strained eye, MLfo(b) For relaxed eye, M LDfo fe D 1 f e 15. (i) Metal : A solid is a conductor if its valence band overlaps its conduction band.CBVB(3)F
Solutions of Sample Question Paper for Class XIIPhysics (Code-A)(ii) Insulator : A solid is an insulator if the valence band and the conduction band do not overlap and are separatedby an energy gap between 3 eV and 6 eV.CBEg ( 3 to 6 eV)VB(iii) Semiconductor : A solid is a semiconductor if the energy gap is much smaller as compared to that in caseof insulators. Band energy gap is in the range (0.1 - 1.0 eV)CBEg ( 0.1 to 1.0 eV)VB16. (a) Here, L 1 H, XL 3142 XL 2 fLf 500 Hz(b) XC 1 C 0.10 F2 fCORC 15 F XC Irms1 212.3 2 fCVrms 0.52 AXC I dl r 0dB , B directed outwards17.4 r 3 I dldB 0 24 r 0Idl 0I B dB 4 r 2 2r IFor half coil B 04rSECTION-CdlrI(4)
Physics (Code-A)Solutions of Sample Question Paper for Class XII18. Capacitance of a conductor is defined as the ratio of charge on it to its potentialQ.VNumerically, capacitance of a conductor is equal to the amount of charge required to raise potential through unity.Numerically, C E 0E VdV d 0C Q 0 A VdQ –Q––––E ––P–––On insertion of a conducting slab, capacitance of the conductor increases as C conducting slab inserted between the plates. 0 A, t is thickness of thed t19. Mobility is defined as the magnitude of drift velocity per unit electric field. e Vd,EVd eE eme e em(a) When temperature of the conductor decreases, e decreases and consequently e decreases.(b) e doesn’t depend upon potential difference and thus it remains unchanged.1 1 1 20. For a lens f v uSince the object is real, u –xvxv xFurther, as the distance between the object and the screen is d,Thus, f v d x Thus, f d x x d x x x 2 dx fd 0d d 2 4fd2For the image to be formed, x should be real, x i.e. 1 4f 0d d 4fWhen d 4f, no image is formed.(5)
Solutions of Sample Question Paper for Class XIIPhysics (Code-A)21. The emission of free electron from metal surface is called electron emission. It can take place through any offollowing physical processes.(i) Thermionic Emission : The release of electron from metal as a result of its temperature, i.e. by heating.(ii) Field emission : It is a kind of electron emission in which a very strong electric field pulls the electron out ofmetal surface.(iii) Photoelectric Emission : It is that kind of electron emission in which light of suitable frequency ejects theelectrons from a metal surface.Photoelectric effect equation KEmax h h 0 .22. Phasor diagram,VL E0VL – VCI0VRVCLCRE E0 sin t VR I0 R, VL I0 X L and VC I0 X CE0 VR2 VL VC 2E0 I0 R 2 X L X C Impedance Z 2R 2 X L XC I2(I0)maxI0 is max, when L C12 fL 2 fCf r12 LC(6)
Physics (Code-A)Solutions of Sample Question Paper for Class XII23. Modes of propagation(i) Ground wave propagation : Travel along curved earth’s surface from transmitter to the receiver.(ii) Sky wave propagation : Radio waves travel skywards and if its frequency is below certain critical frequency(typically 30 MHz). It is returned to the earth by ionosphere.(iii) Space wave : In space wave propagation, radio waves travel in a straight line from transmitting antenna to thereceiving antenna.Sky wave : The sky wave below critical frequency travels from the transmitting antenna to receiving antennavia ionosphere. The ionosphere consists of layers of air molecules which have become positively charged byremoval of electrons by sun’s ultraviolet radiations. On striking the earth, the sky wave bounces back to theionosphere where it is again gradually refracted and returned earthwards as if by reflection. This continuesuntil it is completely attenuated.24. In5626Fe nucleus, there are 26 protons and 30 neutronsMass defect 26mp 30mn m 5626 Fe 0.528461 uBinding energy (mass defect)c2 0.528461 931.5 MeV 492.26 MeV Binding Energy 492.26 MeV 8.76 MeVNucleon56(7)
Solutions of Sample Question Paper for Class XII25. Consider the -decay of9292Physics (Code-A)U238U238 90 Th234 2He4Neutron-proton ratio before -decay Neutron-proton ratio after -decay 238 92 1.58792234 90 1.690Thus, neutron-proton ratio increases during -decay.Nuclear Force : Force between nucleons is called nuclear forces. It is one of the four fundamental forces innature.Properties :(i) It is an attractive force.(ii) It is independent of the interacting nucleons.(iii) It is a short range force.(iv) It is a non-central force.OR(a) En 13.6Z 2 eVn2For Li , Z 3En 122.4eVn2For transition from n 1 to n 3 E E3 E1 hc 114.2 Å(b) No. of spectral lines 326. During solar flare, a large number of electrons and protons are ejected from the sun, some of them get trapped inthe earth’s magnetic field and move in helical paths along the field lines. The magnetic field lines come closer toeach other near the magnetic pole. Hence, density of charges increases near the poles. These particles collidewith atoms and molecules of the atmosphere. Excited oxygen atom emit green light and erected nitrogen atomsemit pink light.(8)
Physics (Code-A)Solutions of Sample Question Paper for Class XIISECTION-D27. (a) Consider point P on the screen, path differenceP x S2 P S1P2d S2 P 2 y D 2 2 S1yd2d S1P y D 2 2 2S2 P 2 S1P 2 2 ydS2 P S1PQSS2D2yd S2P S1P D 2Dyd x DFor constructive Interference, x n n ydDn D n 0,1,. dFor destructive interference y x 2n 1 y2 2n 1 D n 0,1,. 2dHence, fringe width Dd(b) Fresnel distance,ZF a2 ZF 40 mORHuygens Principle : Every point on wavefront may be considered as a source that produces secondary wavelets.These wavelets propagate in the forward direction with a speed equal to speed of wave motion. The surface whichtouches these wavelets at any later instant is the position of new wavefront, called secondary wavefront.AD CE v1v2sin i ADfrom CADCDsin r CEfrom CEDCDsin i AD CD v1 sin r CD CE v 2(9)
Solutions of Sample Question Paper for Class XIIAs vPhysics (Code-A)c v1 2 , v 2 1sin i 2 sin r 1 sini constant (Snell’s law)Convex LensIncident wavefrontRefracted wavefrontIncidentwavefrontRefractedwavefront28. (a) V-I characteristicIF (mA)80MajorityCarriers6040Breakdown20VK (Knee Voltage)VF (V)–80 –60 –40 –20VR (V)–1ReverseBreakdownMinorityCarriers–2Forward BiasIR ( A)PnForward BiasVPnReverse BiasV(b) E ghc 1242 eV nm 0.207 eV 6000 nmSince E E g , the photodiode cannot detect wavelength of 6000 nm.(10)
Physics (Code-A)Solutions of Sample Question Paper for Class XIIOR(a) Transistor as a common - emitter amplifier.Circuit Diagram.ICCIBBRLEBEac inputIVCCVCEac outputVBBOperation :(i) With no signal inputVCE VCC IC RL(ii) With signal applied to the emitter base circuit for positive half cycle, increases the forward bias resultingin an increase in collector current. During negative half cycle, the input signal opposes the forward biasof the input circuit, thereby reducing the emitter and consequently the collector current.dc current gain ICIBV VBE VCC9V(b) IB CC 30 ARBRB 300 103 (as VBE VCC )IC IB 50 30 A 1.5 mA 6VVCE VCC IC RC 9 V 1.5 10 3 A 2 103 29. (a) Magnetic field at a point on Axial line : End on position mBS 0 4 r a 2S mBN 0 4 r a 2–mN mr m4raBP BS BN 0 4 r 2 a 2 22aSince r aBN 0 2M BP 4 r 3 BPMagnetic field at a point on equatorial lineBS m BN 0 2 4 r a 2 m BS 0 2 4 r a 2 PS(11)N
Solutions of Sample Question Paper for Class XIIPhysics (Code-A) 2am BP BN BS 0 2 4 r a 2 3/2r a MBP 0 3 4 r Baxial 2BequatorialC(b) As m T m T ' 'm T T ' 200 KOR(a) Biot Savart’s law, dB the magnetic field at point Pdl 0 Idl sin 4 r 2 dBnet 2dB sin IdB rP 0 Idl sin dBnet 24 r 2 dBnet 2 dBnet Bnet 0 Idl a4 r 2 r2 0Iadl4 r 3 Ix dB 0Ia2r a2 x 2 3/2x 20 A 6 10 4 A(b) Ig 30 div div SShunt requireddBaG 0.015 I 1IgGSAmmeter (12)
Chemistry (Code-A)Solutions of Sample Question Paper for Class XIIChemistry (Theory) - Class XII (Code-A)SOLUTIONSSECTION-A1. 3-Bromo-5-fluoro-3, 5-dimethylheptane2. (i) It is a zero order reaction.[R]02K3. Out of the five electron pairs around the central Br-atom, the two lone pairs are at equatorial positions to minimisethe repulsive interactions. Hence, BrF3 has T-shaped structure.(ii) t1/2 FFBrF4. Dispersed phase – milk fatsDispersion medium – water15. Number of A-atoms per unit cell 8(corners) 18Number of B-atoms per unit cell 6 1 32Hence, the formula of the compound is AB3.6.ABaq. A– (aq.) B (aq.)Initially100At equilibrium0.50.50.5van’t Hoff factor 1 1.57. NaCN is used as a depressant for ZnS and prevents it from coming with froth.8. 6XeF4 12H2O 4Xe 2XeO3 24HF 3O2SECTION-B9. The structural formula of 4-methylpent-3-en-2-one isH3C — C — CHCH3—OC — CH3ODue to the presence of H3C — C — group, it will respond positive to iodoform test.(13)
Solutions of Sample Question Paper for Class XIIChemistry (Code-A)10.Osmotic pressure may be defined as the excess pressure which must be applied to the solution side to justprevent the osmosis. Osmotic pressure is directly proportional to temperature as well as pressure.11. 2Cu2S 3O2 2Cu2O 2SO2Cu2S 2Cu2O 6Cu SO212. The two components of starch are amylose and amylopectin. Amylose is a linear polymer of -D-( )-Glucosewhile amylopectin is heavily branched polymer of -D( )-Glucose.OHI/PReduction13. (a) H—C — (CHOH)4 — CH2 — OH CH3CH2CH2CH2CH2CH3———CHO—CHOn-Hexane(b) (CHOH)4 5(CH3CO)2O (CHOCOCH3)4 5CH3COOHCH2OHCH2OCOCH3GlucosePentacetyl glucose14. H2SO4 H :OSO3H—H H3C — CH2 — O – H H H3C —CH2 — O — HSlow H3C — CH2 H2O——H—H Fast H C CH H SOH — C — C — H :OSO3H 2224H15. (a) Hofmann’s Bromamide reaction:OHeatH3C — C — NH2 Br2 4KOH H3C – NH2 2KBr K2CO3 2H2OCONKCOCO—C2H5–I –KI——CON — CH2CH3CO H /H2O—NHKOH(alc.) –H2O—CO——(b) Gabriel phthalimide reaction:COOH H3C — CH2 — NH2COOH(14)
Chemistry (Code-A)Solutions of Sample Question Paper for Class XII16. (a) Distinction can be made by Lucas reagent. Treat both the solutions separately with Lucas reagent which isa mixture of HCl(g) and anhy. ZnCl2. The compound that shows turbidity instantly is 2-methylpropan-2-olwhile the solution that shows turbidity after 5 minutes of heating, is Butan-1-ol.(b) Distinction can be made by litmus test. Add a few drops of blue litmus separately to the solutions of boththe compounds. The solutions which changes the colour of the blue litmus to red is that of phenol whilethe other is benzyl alcohol.17. (a) Aniline does not undergo Friedel Crafts reaction. It being a Lewis base, coordinates with anhy. AlCl3.—H2N AlCl3The amino group now, is not in a position to activate the benzene ring towards electrophilic substitution,that is alkylation or acylation. Also, AlCl3 does not remain free to generate the carbonation from alkyl oracyl halide.(b) The –NH2 group over benzene ring makes it so electron-rich site that Br2 molecule itself gets polarizedunder its influence. This helps in the generation of the electrophile Br , even in the absence of a halogencarrier.OR(a) During acylation aniline gives CH 3 – C – NH, which is a resonance stabilized compound.O(b) 3 -amine does not contain replaceable hydrogen on N-atom.18. The solution of acetone and chloroform will show negative deviation from their ideal behaviour.Cl ( ) (–)CH3Cl–C–H.O CClCH3There is formation of hydrogen bond between acetone and chloroform molecules, so the escaping tendency ofboth components is lowered.SECTION-C19. We know that, zz MNA a 3, here ‘a’ is edge length6.23 6.023 1023 (4.00 10 8 )3 4.002 460 The cubic unit cell is ‘face-centered cubic’.(15)
Solutions of Sample Question Paper for Class XIINow, in fcc lattice, r Chemistry (Code-A)a2 24 10 82 2 1.414 10–8 cm20. (a) Terylene is a condensation polymer and its monomers are Ethylene glycol (HOCH2CH2OH) and terephthalicacid HOOCCOOH(b) Teflon is an addition polymer and its monomer is tetrafluoroethylene (CF2 CF2)CH3(c) Its monomer unit is isoprene i.e., CH 3–C CH–CH 321. (a) These two compounds are ionization isomers to each other and on treating the aq. solutions of the two withAgNO3(aq), [CO(NH3)5(SO4)]Br gives yellow ppts of AgBr while the other compound does not.(b) The electronic configuration of Fe(Z 26) is [Ar], 3d6, 4s2 while that of Fe(II) is [Ar], 3d6 since F– is aweak field ligand that does not cause pairing up of electrons, sp3d2 hybridization takes place.[FeF6]4–:3d4s4p34d2sp d hybridizationThe structure of the complex is octahedral and it is a high-spin complex. On the other hand, CN– is a strongfield ligand that causes greater crystal field splitting and hence, pairing up of electrons.[Fe(CN)6]4–:3d4s24p3d spThis way, d2sp3 hybridization takes place and the octahedral structure is diamagnetic.22. (a)PhysisorptionChemisorption(i) The adsorbate molecules (i) Adsorbate molecules areare held to the surface ofheld to the surface ofadsorbent by weak vanadsorbent by strongder Waals forces.chemical forces (chemicalbonds).(ii) It is not specific in nature.(ii) It is highly specific.(iii)It forms multimolecular (iii) It forms monomolecularlayers.layers.(16)
Chemistry (Code-A)Solutions of Sample Question Paper for Class XIILyophilic sol(b)Lyophobic sol(i) When dispersion mediumlikes dispersed phase,the sol is called lyophilic.(i) When dispersion mediumdislikes dispersed phase,the sol is called lyophobic.(ii) They are quite stable and (ii) They are easily precipitatedare not precipitated easily.by addition of a smallamount of a suitableelectrolyte.(iii) They are reversible innature.(iii) They are reversible innature.23. (a) ICl is polar due to electronegativity difference between I and Cl. But I2 is nonpolar covalent compound.So stability of polar bond is less than covalent bond and ICl is more reactive than I2.1 odd electron (incomplete octet of N)NOOOO(b)(Complete octet of N)N—NOOAt lower temperature NO2 is converted into N2O4 due to incomplete octet of N in NO2.(c) In H3PO3, oxidation number of P is 3 and in H3PO4, P present in higher oxidation number 5, so H3PO4is not further oxidised.OFFXe24. (a)FF32sp d , Square pyramidalFFPF(b)Triangular bipyramidalFF(c)OHSOOOOSOHOOsp3-Hybridised Sulphur(17)
Solutions of Sample Question Paper for Class XIIChemistry (Code-A)OR(a) Ca 3P2 6H 2O 3Ca(OH) 2 2PH 3Cal. PhosphatePhosphine(b) XeF6 NaF Na [XeF7]– [Sodium heptafluoroxenate (vi)](c) Cu 2H2SO4(conc.) CuSO4 SO2 2H2O25. KP Ae–EP/RT ; P Presence of catalystKA Ae–EA/RT ; A Absence of catalystKP (EP E A )/RTe e E/RT KAKPEEA A Ine log10 10KART2.303RTKPKA (45 55) (EP E A ) Antillog 2.303RT Antilog 2.303R300K KPKA10Antilog 1.0032.303 8.314 300KP K A–26. (a) KCN is ionic compound and generate CN ion in solution, the attack takes place mainly through carbon atomdue to C — C bond strength is higher than C – N bond strength.(b) Resonating structure of chlorobenzeneCI— CI—— CI—— CI———CILone pair of Cl is conjugated with double bonds of ring. So the electron density of ortho and parapositions increases by resonance and it undergoes electrophilic substitution at ortho and para positionswhile Cl is deactivating group. Nu(c) CH2 CH – CH2 – Cl CH2 CH – CH2 ClAllyl ChlorideAllyl (more stable carbocationby resonance) NuCH3 – CH2 – CH2 – Cl CH3 – CH2 – CH2 Cln-Propylchloride1 carbocationIntermediate allyl carbocation is more stable than 1 carbocation. So the allyl chloride is more reactive towardsnucleophilic substitution.(18)
Chemistry (Code-A)Solutions of Sample Question Paper for Class XII27. Deficiency diseases by vitamin – A Xerophthalmia, Night blindnessC Scurvy (Bleeding gums)Vitamin-A RetinolVitamin-C Ascorbic acidSECTION-DOOOHOAldolOHCH3 — CH CH2 — CH CH3 — CH — CH2 — CH CH3 — CH — CH228. (a) (i)LiAlH4(Condensations)Ba(OH)2HCH2 — OHButan-1-3-diolCH3OOMgI CH3 — C — OHH2O(ii) CH3 — C — CH3 CH3 — Mg — I CH3AcetoneMethyl–Mg(OH)ICMag. IodideCH3CH3CH3tert-butyl alcoholAdductCOOHCOOH(Conc.) ( HNO3 H2SO 4) (iii)NO2Meta ntiro benzoic acidBenzoic Acid(b) (i) Clemmensen reductionZn–HgCH3 — CHO 4[H] CH3 — CH3 H2O EthanalEthane(ii) Cannizzaro reaction 2HCHO NaOH CH3 OH HCOONaFormaldehydeMethanolOR(a) (i)Zn–Hg (Conc. HCl)NaCl2/U.V.CH3 — CH CH3 — CH3 CH3 — CH2 — Cl CH3 — CH2 — CH2 — ReactionCl2/U.V.Ethanal(i) B2H6,THFCH3 — CH2 — CH2 — CH2 CH3 — CH2 — CH CH2 CH3 — CH2 — CH — CH3(ii) H O /OH–Butan-1-ol2(CH3)3COK2ClOH(19)
Solutions of Sample Question Paper for Class XIIChemistry (Code-A)HO – CH2CHOCOOHKMnO4[O](ii)CH2 – CH CH2NaOH Cl – CH2 – CH CH2 CaO AlCl3BenzaldehydeB2H6, THF HO223-Phenyl PropanolCH2 – CH3COOHBaeyer’s reagentKMnO4(iii)CH2 – CH2Soda LimeNaOH CaO Ethyl Benzene DecarboxylationBenzoid AcidBenzene(b) (i) Aldol condensationOHOHOBa(OH)2CH3 — CH CH2 — CHO CH3 — CH — CH2 — CHOEthanalAldol(ii) HVZ reactionBrOOBr2/PBr2/PBr2/PCH3 — C — OH CH2 — C — OH CH — C — OH BrBrO29. (a) H e– H2(g)Ecell –pH20.0591 log1 H or –0.118 –0.05911 log1 H [H ] 10–2 mol/L pH 2(b) At anodeH2(g) 2H 2e–At cathode2H 2e– 1/2 O2(I) H2O(l)Net reactionH2(g) 1/2 O2(g) H2O(l)(20)OCBr2 — C — OH
Chemistry (Code-A)Solutions of Sample Question Paper for
(8) Solutions of Sample Question Paper for Class XII Physics (Code-A) 25. Consider the -decay of 238 92U 238 234 4 92U Th He 90 2 Neutron-proton ratio before -decay 238 92 1.587 92 Neutron-proton ratio after -decay 234 90 1.6 90 Thus, neutron-proton ratio increases during -decay.
Physics 20 General College Physics (PHYS 104). Camosun College Physics 20 General Elementary Physics (PHYS 20). Medicine Hat College Physics 20 Physics (ASP 114). NAIT Physics 20 Radiology (Z-HO9 A408). Red River College Physics 20 Physics (PHYS 184). Saskatchewan Polytechnic (SIAST) Physics 20 Physics (PHYS 184). Physics (PHYS 182).
Advanced Placement Physics 1 and Physics 2 are offered at Fredericton High School in a unique configuration over three 90 h courses. (Previously Physics 111, Physics 121 and AP Physics B 120; will now be called Physics 111, Physics 121 and AP Physics 2 120). The content for AP Physics 1 is divided
General Physics: There are two versions of the introductory general physics sequence. Physics 145/146 is intended for students planning no further study in physics. Physics 155/156 is intended for students planning to take upper level physics courses, including physics majors, physics combined majors, 3-2 engineering majors and BBMB majors.
Physics SUMMER 2005 Daniel M. Noval BS, Physics/Engr Physics FALL 2005 Joshua A. Clements BS, Engr Physics WINTER 2006 Benjamin F. Burnett BS, Physics SPRING 2006 Timothy M. Anna BS, Physics Kyle C. Augustson BS, Physics/Computational Physics Attending graduate school at Univer-sity of Colorado, Astrophysics. Connelly S. Barnes HBS .
PHYSICS 249 A Modern Intro to Physics _PIC Physics 248 & Math 234, or consent of instructor; concurrent registration in Physics 307 required. Not open to students who have taken Physics 241; Open to Freshmen. Intended primarily for physics, AMEP, astronomy-physics majors PHYSICS 265 Intro-Medical Ph
strong Ph.D /strong . in Applied Physics strong Ph.D /strong . in Applied Physics with Emphasis on Medical Physics These programs encompass the research areas of Biophysics & Biomedical Physics, Atomic Molecular & Optical Physics, Solid State & Materials Physics, and Medical Physics, in
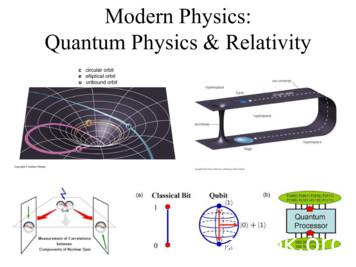
Modern Physics: Quantum Physics & Relativity. You can’t get to Modern Physics without doing Classical Physics! The fundamental laws and principles of Classical Physics are the basis Modern Physics
Ib physics hl ia. Ib physics hl data booklet. Ib physics hl notes. Ib physics hl topics. Ib physics hl textbook. Ib physics hl past papers. Ib physics hl grade boundaries. If you are watching this program, you are probably thinking of taking IB Economics or are currently enrolled in the