BS CHEMISTRY PROGRAM - University Of Turbat
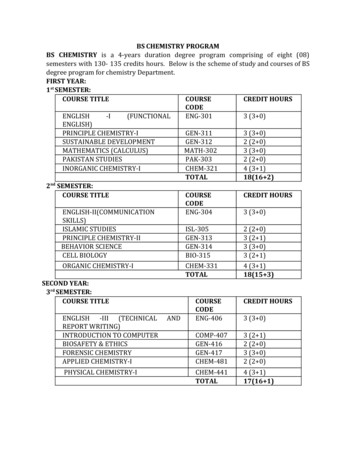
BS CHEMISTRY PROGRAMBS CHEMISTRY is a 4-years duration degree program comprising of eight (08)semesters with 130- 135 credits hours. Below is the scheme of study and courses of BSdegree program for chemistry Department.FIRST YEAR:1 st SEMESTER:COURSE TITLECOURSECREDIT HOURSCODEENGLISH-I(FUNCTIONALENG-3013 (3 0)ENGLISH)PRINCIPLE CHEMISTRY-IGEN-3113 (3 0)SUSTAINABLE DEVELOPMENTGEN-3122 (2 0)MATHEMATICS (CALCULUS)MATH-3023 (3 0)PAKISTAN STUDIESPAK-3032 (2 0)INORGANIC CHEMISTRY-ICHEM-3214 (3 1)TOTAL18(16 2)2 nd SEMESTER:COURSE TITLECOURSECREDIT HOURSCODEENGLISH-II(COMMUNICATIONENG-3043 (3 0)SKILLS)ISLAMIC STUDIESISL-3052 (2 0)PRINCIPLE CHEMISTRY-IIGEN-3133 (2 1)BEHAVIOR SCIENCEGEN-3143 (3 0)CELL BIOLOGYBIO-3153 (2 1)ORGANIC CHEMISTRY-ICHEM-3314 (3 1)TOTAL18(15 3)SECOND YEAR:3 rd SEMESTER:COURSE TITLECOURSECREDIT HOURSCODEENGLISH -III (TECHNICAL ANDENG-4063 (3 0)REPORT WRITING)INTRODUCTION TO COMPUTERCOMP-4073 (2 1)BIOSAFETY & ETHICSGEN-4162 (2 0)FORENSIC CHEMISTRYGEN-4173 (3 0)APPLIED CHEMISTRY-ICHEM-4812 (2 0)PHYSICAL CHEMISTRY-ICHEM-441TOTAL4 (3 1)17(16 1)
4 th SEMESTER:COURSE TITLESTATISTICSMARKETING & MANAGEMENTANALYTICAL CHEMISTRY-IENVIRONMENTAL CHEMISTRY-IBIOCHEMISTRY-ITHIRD YEAR:5 th SEMESTER:COURSE TITLEINORGANIC CHEMISTRY-IIORGANIC CHEMISTRY-IIPHYSICAL CHEMISTRY-IIANALYTICAL CHEMISTRY-II6 th SEMESTER:COURSE TITLEINORGANIC CHEMISTRY-IIIORGANIC CHEMISTRY-IIIPHYSICAL CHEM-532CHEM-542CHEM-552TOTALCREDIT HOURS3 (3 0)3 (3 0)4 (3 1)3 (3 0)4 (3 1)17(15 2)CREDIT HOURS4 (3 1)4 (3 1)4 (3 1)4 (3 1)16(12 REDIT HOURS4 (3 1)4 (3 1)4 (3 1)4 (3 1)16(12 4)FOURTH YEAR:FIELD OF SPECIALIZATIONThe Department of Chemistry, University of Turbat offers specialization in:I)Organic ChemistryII)Inorganic ChemistryIII)Physical ChemistryV)Analytical ChemistryIV)7 th SEMESTER:INORGANIC CHEMISTRYCOURSE TITLEPAPER-I(INORGANICREACTIONMECHANISM)PAPER-II (π- ACEPTOR LIGANDS ANDINORGANIC POLYMERS)BiochemistryCOURSECODECHEM-624CREDIT HOURSCHEM-6253 (3 0)3 (3 0)2
PAPER-III(INORGANICSPECTROSCOPY)INORGANIC LAB-IRESEARCH METHODOLOGYRESEARCH PROJECTCHEM-6263 (3 0)CHEM-627CHEM-6911 (0 1)3 (3 0)3 (0 3)16(12 4)TOTALORGANIC CHEMISTRYCOURSE TITLEPAPER-I(HETEROCYCLICANDORGANOMETALLIC COMPOUNDS)PAPER-II ( REACTIVEINTERMEDIATES)PAPER-III (ORGANIC SPECTROSCOPY)ORGANIC CHEMISTRY LAB-IRESEARCH METHODOLOGYRESEARCH PROJECTCOURSECODECHEM-634CREDIT HOURSCHEM-6353 (3 0)CHEM-636CHEM-637CHEM-6913 (3 0)1 (0 1)3 (3 0)3 (0 3)16(12 4)TOTALPHYSICAL CHEMISTRYCOURSE TITLEPAPER-I (ELECTROCHEMISTRY ANDSTATISTICAL THERMODYNAMICS)PAPER-II (POLYMER CHEMISTRY)(QUANTUMCHEMISTRYANDMOLECULAR SPECTROSCOPY)PHYSICAL CHEMISTRY LAB-IRESEARCH METHODOLOGYRESEARCH PROJECTCOURSECODECHEM-644CREDIT HOURSCHEM-645CHEM-6463 (3 0)3 (3 0)CHEM-647CHEM-6911 (0 1)3 (3 0)3 (0 3)16(12 4)TOTALANALYTICAL CHEMISTRYCOURSE TITLEPAPER-I (ATOMIC SPECTROSCOPY)PAPER-II ELECTROANALYTICALTECHNIQUESPAPER-III (ADVANCED SEPARATIONTECNIQUES)ANALYTICAL CHEMISTRY LAB-IPAPER-IV(RESEARCHMETHODOLOGY)RESEARCH PROJECT3 (3 0)3 (3 0)COURSECODECHEM-654CHEM-655CREDIT HOURSCHEM-6563 (3 0)CHEM-657CHEM-6911 (0 1)3 (3 0)3 (3 0)3 (3 0)3 (0 3)3
BIOCHEMISTRYCOURSE TITLEPAPER-I (BIOMEDICAL CHEMISTRY)PAPER-II ( MOLECULAR BIOLOGY)PAPER-III (PHYSICAL TECHNIQUES INBIOCHEMISTRY)BIOCHEMISTRY LAB-IRESEARCH METHODOLOGYRESEARCH PROJECTTOTAL16(12 4)COURSECODECHEM-663CHEM-664CHEM-665CREDIT HOURSCHEM-666CHEM-6911 (0 1)3 (3 0)3 (0 3)16(12 4)TOTALFOURTH YEAR8 th SEMESTERINORGANIC CHEMISTRYCOURSE TITLEPAPER-IV ( ORGANOMETALLICS)PAPER-V(SYMMETRY ANDMAGNETOCHEMSITRY)PAPER-VI(RADIO AND NUCLEARCHEMISTRY)INORGANIC LAB-IIENVIRONMENTAL CHEMISTRY-IIRESEARCH PROJECT (WRITE-UP)COURSECODECHEM-628CHEM-629CREDITHOURS3 (3 0)3 (3 0)CHEM-62103 (3 0)CHEM-6211CHEM-6721 (0 1)3 (3 0)3 (0 3)16(12 4)TOTALORGANIC CHEMISTRYCOURSE TITLEPAPER-IV (NATURAL PRODUCTS)PAPER-V (ORGANIC SYNTHESIS)PAPER-VI (MEDICINAL CHEMISTRY)ORGANIC CHEMISTRY LAB-IIENVIRONMENTAL CHEMISTRY-IIRESEARCH PROJECT 6311CHEM-672TOTALPHYSICAL CHEMISTRYCOURSE TITLEPAPER-IV (REACTION DYNAMICS)PAPER-VRADIATIONANDPHOTOCHEMISTRY3 (3 0)3 (3 0)3 (3 0)COURSECODECHEM-648CHEM-649CREDITHOURS3 (3 0)3 (3 0)3 (3 0)1 (0 1)3 (3 0)3 (0 3)16(12 4)CREDIT HOURS3 (3 0)3 (3 0)4
PAPER-VI (COLLOID AND SURFACECHEMISTRY)PHYSICAL CHEMISTRY LAB-IIENVIRONMENTAL CHEMISTRY-IIRESEARCH PROJECT L CHEMISTRYCOURSE TITLEPAPER-IV VNUCLEAR NALYTICAL CHEMISTRY LAB-IIENVIRONMENTAL CHEMISTRY-IIRESEARCH PROJECT (WRITE-UP)PAPER- VI (MICROBIOLOGY ANDIMMUNOLOGGY)PAPER-V BIONANOTECHNOLOGYPAPER-VI NUTRITIONAL CHEMISTRYBIOCHEMISTRY LAB-IIENVIRONMENTAL CHEMISTRY-IIRESEARCH PROJECT (WRITE-UP)3 (3 0)3 (0 3)16(12 4)CREDIT HOURSCHEM-6593 (3 0)CHEM-6510CHEM-6511CHEM-6723 (3 0)3 (3 0)1 (0 1)3 (3 0)3 (0 3)16(12 4)COURSECODECHEM-667CREDIT HOURSCHEM-668CHEM-669CHEM-6610CHEM-6723 (3 0)3 (3 0)1 (0 1)TOTALKey code:BIO:CHEM:COMP:ENG:GEN:ISL:MATH:1 (0 1)COURSECODECHEM-658TOTALBIOCHEMISTRYCOURSE TITLE3 (3 0)3 (3 0)3 (3 0)3 (0 3)16(12 4)Biology CourseChemistry courseComputer CourseEnglish CourseGeneral CourseIslamic Studies / EthicsMathematics Course5
PAK:Pakistan StudiesSTAT:Statistics CourseThe key code is followed by three digits; the 1 st digit denotes level of course, the 2 nd digitspecifies the following subject identity:Compulsory Course0General Course1Inorganic Chemistry2Organic Chemistry3Physical Chemistry4Analytical Chemistry5Biochemistry6Environmental Chemistry7Applied Chemistry8Research Methodology9rd th The 3 and 4 digit is used to indicate the number of courses within the specialty.FIRST SEMESTER (1 ST YEAR)INORGANIC CHEMISTRY-I(CHEM-321)(3 1) COURSE OBJECTIVES:Students will acquire knowledge about the key introductory concepts of chemicalbonding, acid-base chemistry, and properties of p- block elements as well asuse this knowledge for qualitative and quantitative analysis of inorganiccompounds during laboratory work. COURSE CONTENTS: Chemical Bonding: Types of chemical bonding, ionic and covalent bonding,localized bond approach, theories of chemical bonding, valance bond theory(VBT), hybridization and resonance, prediction of molecular shapes usingValence Shell Electron Pair Repulsion (VSEPR) model, molecular orbital theory(MOT) applied to diatomic molecules, delocalized approach to bonding,bonding in electron deficient compounds, hydrogen bonding. Acids and Bases: Brief concepts of chemical equilibrium, acids and basesincluding soft and hard acids and bases (SHAB), relative strength of acids andbases, significance of pH, pK a , pK b andbuffer solutions, theory of indicators, solubility, solubility product, common ion effect and their industrialapplications. p-Block Elements: Physical and chemical properties of p-block elements logens,pseudo-halogens and polyhalides.6
Periodic table and periodicity:introduction, electronic configuration and periodic table, classification ofelements on the basis of S, P, d and f valance orbitals, periodicity, shieldingeffect, effective nuclear charge, density of elements, atomic volume, atomic andionic size, ionization energy, electron affinity, electronegativity, application ofelectro negativity, electropositive and electronegative element, characteristicsof elements, polarisibility and polarizing power of ions, periodicity incompound (Halides, Oxides, sulphides and Hydrides) periodicity in transitionelements. PRACTICAL:Lab safety and good laboratory practices, knowledge about material safety data sheets(MSD), disposal of chemical waste and first-aid practices, qualitative analysis ofsalt mixtures, quantitative analysis, acid- base titrations, preparation andstandardization of acid and alkali solutions, redox titrations, preparation andstandardization of potassium permanganate solution and its use for thedetermination of purity of commercial potassium oxalate or oxalic acid,preparation and standardization of sodium thiosulfate solution and its use indetermination of copper in a given sample, gravimetric analysis, determinationof barium in a given sample, determination of chloride in a given solution. RECOMMENDED BOOKS: Shriver, D. F., Atkins, P. W., Langford, C. H., Inorganic Chemistry, 2 nd ed., OxfordUniversity Press, (1994). Cotton, F. A. and Wilkinson, G., Advanced Inorganic Chemistry, 6th ed.,John-Wiley & Sons, New York, (2007). Huheey, J. E., Inorganic Chemistry: Principles of Structure and Reactivity, 3 rd ed., Harper International SI Edition, (2006). House, J. E., Inorganic Chemistry, Academic Press. USA, (2008). Lee, J. D., Concise Inorganic Chemistry, 5 th ed., Chapman and Hall, (1996). Miessler, G. L., Tarr, D. A., Inorganic Chemistry, 3 rd ed., Pearson Education,India, (2008). Huheey, J. E., Kieter E. A., Keiter L. R., Inorganic Chemistry: Principles ofStructure and Reactivity, 4 th ed., Benjamin-Cummings Pub Co., (1993). Sharpe, A. G., Inorganic chemistry, 3 rd ed., Pearson Education India, (1981). Chaudhary S. U., Ilmi Textbook of Inorganic Chemistry, Ilmi Kitab Khana,Lahore, (2013). Catherine E. House croft, Alan G. Sharpe, Inorganic Chemistry, 3 rd ed., PrenticeHall, (2008). Kathleen A. H., James E. H., Descriptive Inorganic Chemistry, 2 nd ed., BrooksCole, (2010). Wulfsberg G., Principles of Descriptive Inorganic Chemistry, 1 st ed., UniversityScience Books, (1991).7
Hill, R. H. JR and Fister, D. C., Laboratory Safety for Chemistry Students,John-Wiley & Sons, Inc., (2010).Mendham, J., Denny, R. C., Barnes, J. D., Thomas, M. and Sivasankar, B., Vogel’sTextbook of Quantitative Chemical Analysis, 6 th ed., Pearson Education, Ltd.,(2000).Svehla, G., Vogel’s Qualitative Inorganic Analysis, 7 th ed., (7 th imp.), PearsonEducation, Ltd., (2009).PRINCIPLES OF CHEMISTRY -I (GEN-311) (3 0) COURSE OBJECTIVES: This subject enables the students to get the knowledge pertaining basic ofChemistry and separation techniques. COURSE CONTENTS: Introduction:Atom, Relative atomic mass, Isotopes, Isobars and Isotones, Determination ofrelative atomic mass (mass spectrophotometer), Empirical formula, Molecularformula, concept of Mole, Avogadro’s number, Stoichiometry, Law of conservation ofmass and Law of definite proportion, Limiting reactant, the concept of Yield(theoretical, actual and percentage yield). Numerical related to the above topics. Solutions:Concept of solutions, Concentration units of the solution, Types of solution, Idealand non-ideal solution, Raoult’s Law, Vapour pressures of solution, Solubility andsolubility curve, Colligative properties of solution, Energetic of solution, Hydrationand Hydrolysis. Numerical related to the above topics. Separation techniques:Introduction to qualitative and quantitative analysis. Filtration (gravity and suctionfiltration), Distillation, Sublimation, Crystallization, Solvent Extraction(Distribution/Partition Law), Chromatography (introduction, classification andpaper chromatography). RECOMMENDED BOOKS Chemistry 4th edition by John A Olmsted and Gregory M. William Chemistry. Student study guide by John A Olmsted. Chemistry Matter and its changes by James E Brady. World of Chemistry by Steven S Zumdahl8
SUSTAINABLE DEVELOPMENT (CHEM-312) (3 0) COURSE OBJECTIVE : The first purpose is to equip students with well-rounded systematic knowledgeand an understanding of key themes of sustainable development within thedevelopment context. These are themes such as the nature of the environmental crisis within theglobal system, national and local sustainable development policy, theories ofsustainable development, the cash between development and conservation aswell as issues in sustainable development practice. The second purpose is to provide students with in-depth knowledge and criticalskills that will enable qualified students to assess and apply strategies andframeworks of sustainable development. In addition, to provide students with the opportunity to study selected themesrelating to gender and gender power relations within the context ofdevelopment by reading and reflecting on texts and case studies dealing withissues such as the feminisation of poverty, urban survival strategies, women asproviders of food security, appropriate technology, gender-sensitive planningand gender policy. COURSE CONTENT: Introduction to Sustainable Development; Global Perspective Culture and Sustainable Development Poverty and Development Gender and Development Governments and International Agreements for Sustainable Development Culture and Sustainable Development What are Sustainable Cities? Global Freshwater Resources The United Nations and Global Sustainability RECOMMENDED BOOKS Ann Swidler and Susan Cotts Watkins (2009). “Teach a man to fish”: thesustainability doctrine and its social consequences. In World Development. Vol.37, Issue 7:1182-1196. Our Common Future: Toward Sustainable Development. UNEP/UNDOdocument available at their website. Priscilla Stone (2003). Is Sustainability for Development Anthropologists. InHuman Organization. Vol. 62, No. 2: 93-99. Sustainable Development Strategy Pakistan, [readings to be assigned] Maia Green (2006). Representing Poverty and Attacking Representations:Perspectives in Poverty from Social Anthropology. E-Quad working paper no.27. Available at: http://192.75.12.210/ids/documents/Q2 WP27 Green.pdf Abhijit Banerjee and Esther Duflo (2007). The Economic Lives of the Poor. InJournal of Economic Perspective, Vol 21, No. 1 :141-167.9
Amertya Sen (1983). Poverty and Famine: Essays on Entitlement andDeprivation. Oxford University Press. [selected chapters] UNDP Human Development Report, MDG and SGD [available at UNDP websites Naila Kabeer (2005). Gender equality and women’s empowerment: a criticalanalysis of the third Millennium Development Goal. In Gender and DevelopmentVol. 13, No. 1:13-24 Maxine Molyneux (2002). Gender and the Silences of Social Capital: Lessonsfrom Latin America. In Development and Change Vol. 33, No. 2:167– 88. Aminur Rahman (1999). Micro-credit Initiatives for Equitable and SustainableDevelopment: Who Pays? In World Development. Vol. 27, No. 1:67-82. Carol Carpenter (2001). The role of economic invisibility in development:veiling women's work in rural Pakistan. In Natural Resources Forum. Vol. 25.Issue. 1: 11-19 UN, “Resilient People, Resilient Planet Report – 2012” (web); UN – “Global Sustainability Panel's Vision” (web) Agyeman, Chapter 1: Introducing just sustainabilities, pp 4 – 34. The Happy Planet Index: 2012 Report: A global index of sustainable wellbeing.(PDF) om/watch?v sZPYI8BfnBs Marks, N et al (2012) Agyeman, Chapter 4, Conclusions Living in Denial, by Kari Marie Norgaard, Introduction and Chapter 6. Bottled Water: the pure commodity in the age of branding, Journal of ConsumerCulture, Richard Wilk (available on PowerCampus) Rees, “The built environment and the ecosphere” (web) Rees, “Getting Serious about Urban Sustainability” (web)ENGLISH I (Functional English) (ENG-301)(3 0) COURSE OBJECTIVES:Enhance language skills and develop critical thinking. COURSE OUTLINES: Comprehension Applied grammar and Usage Introduction to Critical Thinking & Reading Paragraph Development & Organization speaking ,listening , reading and writing skills Story writing and telling Skimming for general idea Scanning for specific information Note makingRECOMMENDED BOOKS:10
Practical English Grammar by A. J. Thomson and A.V. Martinet. Exercises 1. 3 rd Edition. Oxford University Press. 1997. ISBN0194313492 Practical English Grammar by A.J. Thomson and A.V. Martinet.Exercises 2. 3 rdEdition. Oxford University Press. 1997. ISBN0194313506 Intermediate by Marie-Christine Boutin, Suzanne Brinand and Francoise Grellet.Oxford Supplementary Skills. Fourth Impression 1993. ISBN 0 19 435405 7 Pages20-27 and 35-41.Upper Intermediate. Brain Tomlinson and Rod Ellis. Oxford Supplementary Skills.Third Impression 1992. ISBN 0 19 4534022.MATHEMATICS -I(MATH-302) (3 0) COURSE OBJECTIVES:Students will acquire knowledge about various basic materials of math which arevery necessary for understanding physical and analytical chemistry. COURSE CONTENTS: Real number system: Natural number, whole number, integers, prime number,composite number, even number, odd number rational number, recurringdecimal, pure recurring decimal, mixed recurring decimal, irrational number andreal number. Closer, commutative, associative property. Additive, multiplicativeidentity. Additive inverse and multiplicative inverse. Distributive property ofmultiplication, over addition and subtraction. Equality: Properties, reflecsive property, symmetric property, transitiveproperty, multiplicative property, cancellation property, w.r.t multiplication,additive property, cannulation property w.r.t addition. Inequality: Properties of inequality of real number, tricholomy property,transitive property, additive property, multiplicative property. Decimal: Properties in working with decimal, addition, subtraction,multiplication, division. RECOMMENDED BOOKS: Frank A.Jr, Elliott Mendelson, Calculus, Schaum’s Outline Series, 4th edition,1999. H. Anton, I. Bevens, S. Davis, Calculus, 8th edition, Jhon Willey & Sons, Inc. 2005. Hughes-Hallett, Gleason, McCallum, et al, Calculus Single and Multivariable, 3rdEdition. John Wiley & Sons, Inc. 2002. Thomas, Calculus, 11th Edition. Addison Wesley publishing company, 2005.PAKISTAN STUDIES (PAK-303)(2 0) COURSE OBJECTIVES:To take an analytical view in the history and development of Muslim society andculture in the sub-continent, emergence of Pakistan and its constitutionaldevelopment. To develop an appreciation of the issues and challenges currently11
being faced in Pakistan. The strengths of its people and strategies to deal with theimpediments to progress. International relations of Pakistan. COURSE CONTENTS:Historical background of Pakistan: Muslim society in Indo-Pakistan, the movementled by the societies, the downfall of Islamic society, the establishment of BritishRaj- Causes and consequences. Political evolution of Muslims in the twentiethcentury: Sir Syed Ahmed Khan; Muslim League; Nehru; Allama Iqbal:Independence Movement; Lahore Resolution; Pakistan culture and society,Constitutional and Administrative issues, Pakistan and its geo-political dimension,Pakistan and International Affairs, Pakistan and the challenges ahead. RECOMMENDED BOOKS: Afzal, M. Rafique. Political Parties in Pakistan, Vol. I, II & III. Islamabad: NationalInstitute of Historical and cultural Research, 1998. Akbar, S. Zaidi. Issue in Pakistan’s Economy. Karachi: Oxford University Press,2000. Amin, Tahir. Ethno - National Movement in Pakistan, Islamabad: Institute of PolicyStudies, Islamabad. Burki, Shahid Javed. State & Society in Pakistan, The Macmillan Press Ltd 1980. Mehmood, Safdar. Pakistan Kayyun Toota, Lahore: Idara-e-Saqafat-e- Islamia, ClubRoad, nd. Mehmood, Safdar. Pakistan Political Roots & Development. Lahore, 1994. Muhammad Waseem, Pakistan Under Martial Law, Lahore: Vanguard, 1987. 14.Haq, Noor ul. Making of Pakistan: The Military Perspective. Islamabad: NationalCommission on Historical and Cultural Research, 1993. Wilcox, Wayne.The Emergence of Banglades., Washington: American Enterprise,Institute of Public Policy Research, 1972. Zahid, Ansar. History & Culture of Sindh. Karachi:Royal Book Company, 1980SECOND SEMESTER (1 ST YEAR)ENGLISH-II (Communication skill) (ENG-304)(3 0) Course Objectives : Write & speak clearly, concisely, and convincingly. Create impressive formal & informal presentations that are delivered withconfidence and poise. Develop and
The Department of Chemistry, University of Turbat offers specialization in: I) Organic Chemistry II) Inorganic Chemistry III) Physical Chemistry IV) Biochemistry V) Analytical Chemistry 7 t h SEMESTER: INORGANIC CHEMISTRY COURSE TITLE COURSE CODE CREDIT HOURS PAPER-I (INORGANIC REACTION MECHANISM)
Chemistry ORU CH 210 Organic Chemistry I CHE 211 1,3 Chemistry OSU-OKC CH 210 Organic Chemistry I CHEM 2055 1,3,5 Chemistry OU CH 210 Organic Chemistry I CHEM 3064 1 Chemistry RCC CH 210 Organic Chemistry I CHEM 2115 1,3,5 Chemistry RSC CH 210 Organic Chemistry I CHEM 2103 1,3 Chemistry RSC CH 210 Organic Chemistry I CHEM 2112 1,3
Physical chemistry: Equilibria Physical chemistry: Reaction kinetics Inorganic chemistry: The Periodic Table: chemical periodicity Inorganic chemistry: Group 2 Inorganic chemistry: Group 17 Inorganic chemistry: An introduction to the chemistry of transition elements Inorganic chemistry: Nitrogen and sulfur Organic chemistry: Introductory topics
CHEM 0350 Organic Chemistry 1 CHEM 0360 Organic Chemistry 1 CHEM 0500 Inorganic Chemistry 1 CHEM 1140 Physical Chemistry: Quantum Chemistry 1 1 . Chemistry at Brown equivalent or greater in scope and scale to work the studen
Accelerated Chemistry I and Accelerated Chemistry Lab I and Accelerated Chemistry II and Accelerated Chemistry Lab II (preferred sequence) CHEM 102 & CHEM 103 & CHEM 104 & CHEM 105 General Chemistry I and General Chemistry Lab I and General Chemistry II and General Chemistry Lab II (with advisor approval) Organic chemistry, select from: 9-10
From one of these Colorado public four-year institutions Adams State University [B.S. Chemistry] Colorado Mesa University [B.S. Chemistry] Colorado State University-Ft Collins [B.S. Chemistry] Colorado State University-Pueblo [B.S. Chemistry] Fort Lewis College [B.S. Chemistry; Chemistry option] Metropolitan State University of Denver
Chemistry is the science that describes matter, its properties, the changes it undergoes, and the energy changes that accompany those processes. Inorganic chemistry Organic chemistry Physical chemistry Biochemistry Applied Chemistry: Analytical chemistry, Pharmaceutical Chemistry, . Istv an Szalai (E otv os University) Lecture 1 6 / 45
Chemistry of Cycloalkanes 13. Chemistry of Alkyl halides 14. Alcohols 15. Chemistry of Ethers and Epoxides 16. Chemistry of Benzene and Aromaticity 17. Chemistry of Aryl Halides 18. Aromatic Sulphonic Acids 19. Chemistry of Aldehydes and Ketones 20. Carboxylic Acids 21. Chemistry of Carboxylic Acid Derivativ
ADVANCED DIPLOMA Diploma in Chemistry 60% in Analytical Chemistry 3 Theory & Practical, Chemical Quality Assurance, Mathematics 2 Chemical Industrial 1 or S5 Subjects and Chemistry project II. Semester 1 Analytical Chemistry IV Physical Chemistry IV Research Methodology in Chemistry Semester 2 Inorganic Chemistry IV Organic Chemistry IV .