Who Consultative Meeting On High Maximum Containment-PDF Free Download
Special Report on Electrical Standards Report on the 17 Session Of the Consultative Committee On Electricity Volume 92 Number 1 January-Februaiy 1987 B. N. Taylor National Bureau of Standards Gaithersburg, MD 20899 This report provides the background for and sununarizes the main results of the 17th session of the Consultative Com-
Meeting report Highlights and conclusions from the technical consultative meeting on novel coronavirus infection, Cairo, Egypt, 14–16 January 2013 C. Joseph,1 M.R. Malik,2 A.W. Mounts,1 A. R. Mafi,2 S. Briand,1 Z.A. Memish 3,4 and the technical working group for the meeting on novel coronavirus
P. SCHAVEMAKER. 5 During last Core Consultative Group 28/06 meeting, Core TSOs indicated that they will come back to Market Parties regarding the questions below on Transparency and RAO RAO l W
A. OPENING (Agenda item 1) 1. The 1st meeting of the Tripartite Joint Competition Authority (JCA) took place on the 31st May and 1st June, 2011 at the Cresta Lodge, Gaborone, Botswana. The meeting was preceded by a consultative meeting of the Air Transport experts from COMESA, EAC and SADC Secretariats and T
untuk melakukan uji coba dapat menggunakan pedoman yang tercantum dalam consultative paper ini dalam melakukan perhitungan. Uji coba akan dilakukan untuk data triwulanan, yaitu untuk posisi data Desember 2016, Maret 2017, Juni 2017, dan September 2017. Hasil uji coba
THIRD CONSULTATIVE REPORT ON CPDS Page 2 2. RATIONALE Stakeholder consultation is one of the critical steps in developing a policy as it ensures that every practical and viable policy alternative has been considered. Stakeholders and those closest to a problem or affected by policy changes can sometimes suggest useful ways to
SOCIAL SECURITY MEMORANDUM Date: August 22, 2003 Refer To: To: James F. Martin Regional Commissioner Chicago From: Assistant Inspector General for Audit Subject: Use of Mental Consultative Examinations by the Wisconsin Disability Determination Bureau (A-01-03-23090) Our objective was to determine whether the Wisconsin Disability Determination Bureau
Michigan Community Services, Inc. MCSI Deaf Services Community Living Supports P.O. Box 317 Swartz Creek, MI 48473-0317 810 635 4407 mcsionline.org Resident Advancement, Inc. Resident Advancement CLS P.O. Box 555 Fenton, MI 48430-0555 810 750 0382 Consultative Services Genesee Health System Consultative - LLP 1057 Coldwater Road Flint, MI 48458
Not all negotiation can be consultative, but it puts you in a very positive position if it can be and is often an automatic outcome when you advise at the tender preparation stage. Consultative negotiation is particularly relevant when you are seeking to build partnerships and establish consortia. 7 Negotiation Briefing 2:Layout 1 28/1/08 15:00 .
Gold Coast Airport - ANACC Minutes - October 2014 Page 1 AIRCRAFT NOISE ABATEMENT CONSULTATIVE COMMITTEE ANACC MINUTES Date: Thursday 30th October 2014 Time: 09.00 - 12.00 Location: The Visions Room - Twin Towns Resort Present Brett Curtis (Chairman) Manager Operations and Standards - GCAPL Carla Golar (Minutes) Manager Risk and Regulatory Compliance - GCAPL
To be effective and successful today sales people need highly developed consultative selling skills. Such skills are foreign to the "professional visitor" who applies standard techniques to opening, presenting, handling objections and closing calls. These are the techniques of product-oriented, or vendor, selling.
MEETING MINUTES 1 Name: Vehicle Services Business Process and Communications Meeting Meeting Date: Wednesday, December 2, 2020 Organizer: Craig Plummer Meeting Time: 10:00 am – 11:00 am Location: Join Microsoft Teams Meeting 1 651-395-7448 United States, St. Paul (Toll) Conference ID: 296 240 105# Purpose of the Meeting
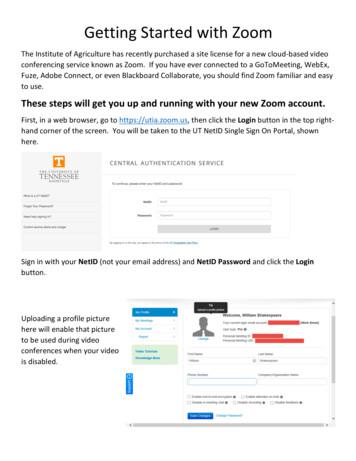
Once logged in, you will be presented with the main Zoom window. At this point you can: Click on Screen Share Meeting to start a meeting sharing your desktop or application. Click on Video Meeting to start a video meeting. Click on Schedule Meeting to setup a future meeting. Click on Join a Meeting to
Meeting #6:Self-Esteem Pages 30-31 Meeting #7: Bullying Page 32 Meeting #8:Gender Inequality Pages 33-34 Meeting #9: Sexual Violence 101 Pages 35-37 Meeting #10: Sexual Assault Exams Pages 38-39 Meeting #11:Self-Care and Assertiveness Pages 40-42 Meeting #12:Safe Places and P
1. In the meeting request, on the Meeting tab, in the Online Meeting group, click Meeting Options. 2. In the Online Meeting Options dialog box, ustomize access and presenters for this meeting check box. 3. Make changes to Access and Presenters Start an unscheduled meeting 1. In the Lync main window, click the Show Menu arrow , and then click .
Chris Berry SP Power Systems Claire Maxim E.ON UK Patrick Hynes NGC The final agreed ToRs are attached as Appendix 2. The working group has met 9 times, on: 1st Meeting 23 September 2003 2nd Meeting 14 October 2003 3rd Meeting 1 December 2003 4th Meeting 12 May 2004 5th Meeting 18 June 2004 6th Meeting 26 August 2004 7th Meeting 12 October 2004
Conduct of the Meeting . Meeting Script and Rules of Conduct. Q: Will a company need to make significant updates to its annual meeting script if it is hosting a virtual rather than an in-person annual meeting? A: Generally, no. The script for a virtual annual meeting should be largely the same as for an in-person meeting.
Fifth Global Meeting of Chairs and Secretariats of Regional Consultative Processes on Migration (RCPs) Exploring Contemporary Migration Challenges: Reflecting on the Outcomes of the 2013 High-level Dialogue on International Migration and Development and the Post-2015 Development Agenda 21–22 October 2015 C
Get Started with Zoom at the U . Page 9 of 10 . In-Meeting Controls . Once you have started or joined a meeting, you can access the meeting controls located at the bottom of the meeting window (move your mouse in the Zoom window to display meeting controls). Learn more about meeting controls for hosts, co-hosts, and attendees. You can also join .
Minutes of 2021 Annual Shareholders’ Meeting will be available on TSMC’s website . - 2 - 2.Meeting Agenda - 3 - Taiwan Semiconductor Manufacturing Company Limited 2021 Annual Shareholders’ Meeting Meeting Agenda (Translation) Time: 9:00 a.m., June 8, 2021 PlaceAmbassador Hotel Hsinchu : . The
Oct 06, 2019 · their first meeting together was a prayer meeting. The word Prayer – proseuche, means toward God – communication which addresses God. The first gathered meeting of believers who would birth the church, was a 10-day “toward God” meeting. The upper room prayer meeting challenges us to the core
o Galion HS CCP Meeting o Ashland HS CCP Meeting o Clear Fork Middle school presentation o Meeting with University of Findlay o EHOVE CCP Meeting o Monroeville HS CCP Meeting o Goal Digital Academy CCP Meeting o Pioneer Day o 16 Admission Interviews Accomplishments STUDENT C
EXHIBITOR BREAKFAST 7:30-8:30 am Founder's Room, Hilton Hotel ANSI N13.1 REVISION 9:00 am-Noon University A (MC) LEADERSHIP MEETING 11:00 am-Noon Dane Room (CC) AEC/STUDENT BRANCH MEETING Noon-1:00 pm Meeting Room L (CC) CONTINUING EDUCATION COMMITTEE Noon-1:00 pm Meeting Room R (CC) SOCIETY SUPPORT COMMITTEE Noon-2:00 pm Meeting Room M (CC)
New York Quarterly Meeting, Religious Society of Friends Meeting for Worship with a Concern for Business Zoom/video-conferencing / should be in Prospect Park @ Quaker Cemetery seventh month and seventeenth day, 2021 ADVANCE AGENDA 2021-06-30 in-progress 11-12pm: Meeting for Worship: Open Meeting for Zoom worship - Zoom link TBA
City Council Work Session Meeting Minutes for April 16, 2020 6.c . City Council Meeting Minutes for May 18, 2020 The minutes of the City Council meeting are enclosed for review. Please note, the minutes for March 16, 2020 meeting were delayed due to technical audio issues during this first meeting utilizing virtual meeting technology.
Creating a Webex Meeting (from a UMB PC) 1. Login to MS Outlook 2. Open your Calendar 3. Begin scheduling a normal meeting 4. Click on . Add Webex Meeting . from the toolbar 5. Click SAVE ( , located in the upper left corner) 1. to see Webex Meeting details when the below text appears. Click here for . new. Meeting and . existing. Meetings
Note: The Unified Meeting 5 desktop client will launch the next time you start or join a meeting. Mozilla Firefox Enabling the Unified Meeting 5 Plug-In before a meeting 1. Open Mozilla Firefox 2. In the menu bar, click Tools Add-Ons 3. Click Plugins 4. Scroll to the Unified Meeting 5 plug-in and select Always Activate in its dropdown menu
Cisco Webex Meeting Server (CWMS) 0. 9. Cisco Webex Meeting Server (CWMS) CWMS IRP On-Premises. Remote Cisco Meeting Server (CMS) / Meeting Management (CMM) On-Premises. Expressway MRA. 1. Cisco Meeting Server (CMS) 1. 1 . Expressway MRA is an optional feature.
On the day before your course, you will receive your Joining Instructions which will contain the Meeting Link along with the Meeting ID and Meeting Password. You can join your course by either: 1. Clicking on the link in your email to join the meeting 2. Opening the app and then entering the Meeting ID and Meeting Password (as shown below) Step 1
List of Participants (Final) - Global Meeting of Chairs and Secretariats of Regional Consultative Processes on Migration (RCPs) Author: International Organization for Migration (IOM) Created Date: 2/13/2012 12:10:06 PM
Notice of the ARD Committee Meeting 19 Be sure to promptly reply to the meeting notice If you cannot attend, you can ask the district to reschedule the meeting to another date/time that will work for both you and the school You may want to give the school some alternate dates and times when you can attend. Notice of the ARD Committee Meeting 20 Be sure to: Confirm that your .
Booking a Meeting Room in Outlook 2010 8/13/12 2. Create a new meeting request by clicking on New Meeting button, located on the “Home” tab of the Ribbon 3. Use the dropdowns underneath the location to select a start and end time/date for the meeting.
Meeting Guide The Supplier . In your Meetings tab, click on the green camera button. This will open the video call window. Click on the add people icon - - in the bottom left hand corner. Copy the unique link for this meeting and send, with the meeting details and day/time, to your colleague. They can join the meeting by pasting this link .
Washington State Liquor and Cannabis Board Meeting Wednesday, November 18, 2020, 10:00am This Meeting was Held via Conference Call Meeting Minutes 1. CALL TO ORDER Chair Jane Rushford called the regular meeting of the Washington State Liquor and Cannabis Board to order at 10:00am on Wednesday, November 18, 2020.
How do we design and structure our fall risk reduction team meeting? Formal meeting agenda Meeting minutes Starting and ending on time Meeting leader University of Nebraska Medical Center. 14 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Had a meeting . Pharmacy Physician Dietician Housekeeping IT .
24TH ANNUAL INTERNATIONAL MEETING This year’s full-meeting pricing includes Advanced Practice Day (formerly known as Physician-Only Day) and one banquet ticket for all meeting attendees. Short on time? Take advantage of our new daily rates. FULL MEETING (Oct. 17–19) SINGLE DAY On o
CCSD Legislation Status Report- 04/02/2021 Bill # Description Sponsors Status Location Last Meeting Title Last Meeting Action Last Meeting Date Next Meeting Title Next Meeting Date AB3 Revises provisions concerning the electronic transmission of certain maps and other documents relat
1.1.2 The Chairman may, unless dissented to or objected by the majority of Directors present at a Meeting at which a Quorum is present, adjourn the Meeting for any reason, at any stage of the Meeting. 1.2 Time, Place, Mode and Serial Number of Meeting 1.2.1 Every Meeting shall have a serial number. 1.2.2 A
the meeting notice if, prior to recessing, the public body specifies the date, time and place for continuation of the meeting, and, immediately following the recessed meeting, posts notice of the date, time and place for the reconvened meeting on or near the door of the place where the original meeting was
May 10, 2021 · MEETING . Monday, May 10, 2021 - 9:00 A.M. VIA TELECONFERENCE . Note on meeting location and format: This public meeting will take place by teleconference. Members of the public can listen to the meeting by dialing phone number 347-921-5612, and entering passcode 672 353 961 #. Once prepared, an audio recording and written transcript of the