Development Of A Redox Free Mitsunobu Reaction Exploiting-PDF Free Download
ii. acid–base neutralization reactions iii. oxidation–reduction or redox reactions. Q.3. What are the important aspects of redox reactions? Ans: Almost every element participate in redox reactions. The important aspects of redox reactions are as follows: i. Large number of natural, biological and industrial processes involve redox reactions .
CHAPTER 12: Redox Reactions . Goals of Chapter: - Understand redox reactions in detail - Review oxidation numbers - Learn electrochemical techniques Application of Redox Chemistry - extracting metals from ores, e.g. Need to learn to balance tricky redox reactions Cu 2CO 3(OH) 2(s) C(s) Æ2Cu(s) 2CO 2(g) H 2O(g .
Recent advances in development of organic and organometallic redox shuttles for lithium-ion redox flow batteries Thuan-Nguyen Pham-Truong[b], Qing Wang[c], Jalal Ghilane*[a] and Hyacinthe Randriamahazaka*[a] Abstract: In the recent years, redox flow batteries especially lithium (RFBs) and derivatives have attracted a wide attention from academia to
OCR Chemistry A H432 Redox and Electrode Potentials p. 3 Method 2: Constructing redox equations using oxidation numbers We can balance a redox equation using oxid
DOI: 10.1002/ejic.201600908 Essay Redox-Active Anticancer Complexes Redox-Active Metal Complexes for Anticancer Therapy Pingyu Zhang[a] and Peter J. Sadler*[a] Abstract: The redox properties of both
Scaffold : Redox Reactions Example Worksheet ATTENTION: This worksheet will be used to play the Redox relay and is modified into cards To determine if a reaction is a REDOX reaction: 1. Assign oxidation numbers to each element in the reaction. 2. If any of the oxidation numbers change in the reaction then the reaction is REDOX. Practice:
Foreign exchange rate Free Free Free Free Free Free Free Free Free Free Free Free Free Free Free SMS Banking Daily Weekly Monthly. in USD or in other foreign currencies in VND . IDD rates min. VND 85,000 Annual Rental Fee12 Locker size Small Locker size Medium Locker size Large Rental Deposit12,13 Lock replacement
PRACTICE PACKET: ELECTROCHEMISTRY 6 LESSON 2: Identifying a Redox Reaction A redox reaction is a reaction in which electrons are transferred from one element to another. The term redox comes from two words, “oxidation” and “reduction.”If something is oxidized, it “burns” in oxygen, as
been investigated by Wang etal. (2012). An intrinsic, self‐ sustained circadian oscillation in SCN redox couples was detected. That novel study also found that redox oscillation could regulate SCN neuronal excitability through non‐tran-sc
Quoting Dr. Gary Samuelson, the medical physicist who created the technology to identify and stabilize reactive Redox signaling molecules, Redox signaling is a fundamental process inside our body that reduces and oxidizes (redoxs) the salt water and biomolecules inside u
The above example described a single-replacement reaction. All reactions of this kind are redox . A series of reactions are attempted. For each attempt, it is recorded whether a reaction occurs or not. . Worksheet 1. The following reactions were performed. Construct a redox table.
demand charges and four hour capacity, it is clear the industry is continuously moving towards energy storage with longer and longer duration. . Vanadium Redox, the most common redox flow battery technology on the market, uses the oxidation states of vanadium. Vanadium redox batteries are limited by the high cost of vanadium and by the
Redox Worksheet Base your answers to questions 31 through 33 on the diagram below. The diagram shows a voltaic cell with copper and aluminum electrodes immediately after the external circuit is completed. 31. Explain the function of the salt bridge. 32. Balance the redox equation below, using the small est whole-number coefficients.
Charges shown apply to the Orange home phone and second line service for home ultra day evening weekend day evening weekend UK landline-Free Free Free Free Free Free UK mobile (excluding 3 mobile)-12.47 7.46 6.90 12.47 7.46 6.90 Orange mobile-12.47 7.46 6.90 12.47 7.46 6.90 3 mobile-21.50 15.20 6.90 21.50 15.20 6.90 0800-Free Free Free Free Free Free 0845-4.50 2.50 2.50 4.50 2.50 2.50
Nov 06, 2014 · bingo bingo bingo bingo large rectangle number 41 anchor 1 anchor 2 any three corners martini glass free free free free free free free free free free free free 9 revised 11/6/2014 2nd chance coverall bingo small ro
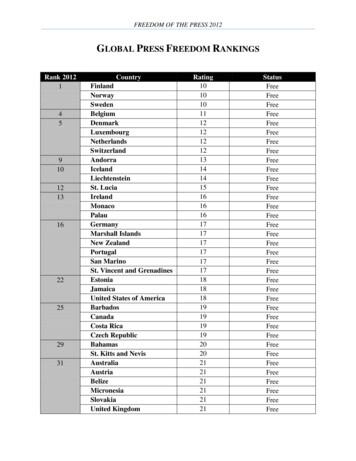
FREEDOM OF THE PRESS 2012 GLOBAL PRESS FREEDOM RANKINGS Rank 2012 Country Rating Status 1 Finland 10 Free Norway Free10 Sweden 10 Free 4 Belgium 11 Free 5 Denmark 12 Free Luxembourg Free12 Netherlands Free12 Switzerland Free12 9 Andorra 13 Free 10 Iceland 14 Free Liechtenstein 14 Free 12 St. Lucia 15 Free 13 Ireland 16 Free Monaco 16 Free Palau 16 Free
(oxidation state 3) is more oxidized than elemental Fe (oxidation state 0). Reduction and oxidation reactions (together known as redox) involve the transfer of electrons (e-) from one molecule to another. Since free electrons cannot exist in solution, these reactions are always coupled. Generally, electrons are transferred from more reduced
2 O ð2 Þ Overall reaction . 2 O ð5Þ 2V 2 þ þ VO 2 þ þ 4H þ . (acac) 3 About 20% at about 2 mA cm 2 Low coulombic and voltage efficiencies [14] Table 1. Membrane-free redox flow batte
akuntansi musyarakah (sak no 106) Ayat tentang Musyarakah (Q.S. 39; 29) لًََّز ãَ åِاَ óِ îَخظَْ ó Þَْ ë Þٍجُزَِ ß ا äًَّ àَط لًَّجُرَ íَ åَ îظُِ Ûاَش
Collectively make tawbah to Allāh S so that you may acquire falāḥ [of this world and the Hereafter]. (24:31) The one who repents also becomes the beloved of Allāh S, Âَْ Èِﺑاﻮَّﺘﻟاَّﺐُّ ßُِ çﻪَّٰﻠﻟانَّاِ Verily, Allāh S loves those who are most repenting. (2:22
76 Lactic Acid Bacteria – R & D for Food, Health and Livestock Purposes Figure 1. E Standard reduction potential 0' h (mV) of some important half-reactions involved in biological processes at 25 C and pH 7. 2.2. Measurement of Eh The first technique for measuring Eh is based on the use of coloured indicators (redox indicators), which are mostly indophenols or indigo
0.01M-V(acac) 3 in 0.5M-TEABF 4: H type cell test OCV : 2.2V, large ohmic voltage drop, coulombic efficiency : 32 47% (50% SOC) Solubility of V(acac) 3 in non-aqueous electrolyte : 1M 0.01M-V(acac) 3 in 0.5M-TEABF 4: H type cell test OCV : 2.2V, large ohmic voltage drop, coulombic efficiency
Here at Redox, many of us are fans of Clayton Christensen’s “Jobs to Be Done” theory of innovation . JTBD posits that all products and services are purchased to “do a job”.
Lecture 11. Redox Chemistry Many elements in the periodic table can exist in more than one oxidation state. Oxidation states are indicated by Roman numerals in parentheses (e.g. ( I), (-IV) etc.). The oxidation state represents the “e
Lecture 11 Diagenesis, Redox Chemistry Organic C Preservation (1) Is seawater normally an oxidizing or reducin
Redox Chemistry Review I. Oxidation State or Number The oxidation state or number of a compound gives a relative measure of how oxidized (electron-poor) or reduced (electron-rich) a compound is.The number is rela
Student Worksheet for Chemical Redox Reactions Attempt to work the following practice problems after working through the sample problems in the videos. Answers are given on the last page(s). Rules for Oxidation Numbers: 1. Oxygen always has an oxidation number of -2, except in peroxides Th
Deicer—a chemical substance used to prevent the formation of ice. . used to predict peak flows. Redox—an oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of . (rain or melted snow) that runs
rarely provide conclusive evidence for the formal oxidation state or, indeed, even the formula unit of a given MOF.[1,11] Strikingly then, the effect of ligand charge state on the electronic and spin interactions in these materials is still poorly understood. Scheme 1. (a) Lewis structures of HOTP6– and the redox sequence on a
UNIT 6 – REDOX REACTIONS 6 The oxidation number of an atom is the charge that would exist on an individual atom if the bonding were completely ionic In simple ions, the oxidation number of the atom is the charge on the ion: - Na , K , H all have an oxidation number of 1 - Mg2 , Ca2 , Pb2 all have an oxidation number of 2 - Cl-, B
The Major Classes of Chemical Reactions. 4.6 Elements in Redox Reactions 4.1 The Role of Water as a Solvent 4.2 Writing Equations for Aqueous Ionic Reactions 4.3 Precipitation Reactions 4.4 Acid -Base Reactions. 4.5 Oxidation -Reduction (Redox) Reactions 4.7
9.2 Redox and Nonredox Chemical Reactions 9.3 Terminology Associated with Redox Processes 9.4 Collision Theory and Chemical Reactions 9.5 Exothermic and Endothermic Chemical Reactions 9.6 Factors That Influence Chemical Reaction Rates 9.7 Chemical Equilibrium 9.8 Equilibrium Constant
almost always involve coupled transfers of electrons from . Repetitively coupled reduction and oxidation reactions, often involving oxygen and reactive oxygen species . A thorough knowledge of the chemistry of redox reactions as well as the biological context will therefore be essential for the understanding of
transforming the electron donating Fc moiety to the more elec-tron withdrawing Fc state. The alkene moiety of 1 is electron rich due to the electron releasing nature of Fc unit. Indeed, the Alfrey–Price e parameter (a semi-empirical measure of electron rich
signs of aging appear. Genetics, age, environment, stress—so many factors can make your skin less vibrant. Redox exclusive anti-aging blends help you get you best skin back. Active cellular communication is essential for healthy, beautiful skin. And ASEA’s patented redox technology is the be
Chemical Redox Agents for Organometallic Chemistry Neil G. Connelly*,† and William E. Geiger*,‡ School of Chemistry, University of Bristol, U.K., and Department of Chemistry, University of Vermont, Burlington, Vermont 05405-0125 Received October 3, 1995 (Revised Manuscri
redox agents in organometallic chemistry. 4 Apart from their redox properties and accompanying use in various oxido-reduction processes, ferrocenium salts can act also as weak Lewis acids, although there are only a few examples of this application. This review gives an overview of applications of ferrocenium ions in organic synthesis.
one-electron redox shuttles so that strategies may be deve-loped to better direct reactivity of potential perfluoroalkyl organometallic intermediates of a catalytic cycle, especially consideringthe factthattrifluorom
TOWARDS REDOX CONTROL OF ORGANOMETALLIC CATALYSTS BEARING FERROCENE-BASED LIGANDS BY SHUOREN DU 杜硕人 OCTOBER 2013 Department of Chemistry Imperial College London A thesis submitted in partial fulfillment of the requirements for the award . Electrochemistry and Chemica
redox-sensitive delivery systems. The design and fabrication of nanoparticles responsive to Glutathione (GSH) can be a promising approach for targeting drug delivery [33]. The GSH reduction is a well-known redox system within cancer cells. On one hand, concentrations of GSH in blood and normal extracellular matrices are reported to be 2-20 μM, at