Welcome To Chem 253
Chem 253, UC, BerkeleyWelcome to Chem mlChem 253, UC, BerkeleyChemistry 253 A & BMaterials & Solid State ChemistryLecture: Tu. Thurs. 12:30-2:00 pm @ 425 LatimerInstructor:Professor Peidong Yang, B68 Hild.,Office Hours:Friday 10:00-12:00pm or by appointmentp yang@berkeley.eduTA:Michael Mooremichael.moore@berkeley.eduChong Liuchongliu@berkeley.edu1
Chem 253, UC, BerkeleyGrading:Problem sets (2)Final40%60%Description:Structure and structure determination of crystalline solids;Solid State SynthesisSolid State CharacterizationElectronic band structure;Chemical bonding in solids;Structure-property relationships.Chem 253, UC, BerkeleyMain Reference BooksWest: Basic solid state chemistry (Wiley, 1999).West: Solid State Chemistry and its Applications (Wiley, 1988)Gersten and Smith: The Physics and Chemistry of Materials (Wiley,2001)Hoffmann: Solids and Surfaces (VCH, 1988)Burdett: Chemical Bonding in Solids (Oxford 1995)Kittel: Introduction to solid State Physics (Wiley, 1996)Ashcroft and Mermin: Solid State Physics (Saunders College, latestedition)Cheetham and Day: Solid State Chemistry: Techniques (Oxford, 1987)Cheetham and Day: Solid State Chemistry: Compounds (Oxford, 1992)Cox: The Electronic Structure and Chemistry of Solids (Oxford, 1987)Wells: Structural Inorganic Chemistry (Clarendon Press, 1984)Wold and Dwight: Solid State Chemistry (Chapman Hall, 1993)2
Chem 253, UC, BerkeleyMaterials Chemistry I1).2).3).4).5).6).7).8).9).10).No LectureBravais Lattice, Reciprocal LatticeXRD, Structural FactorDiffractionDescriptive crystal chemistryDescriptive crystal chemistryCrystal Chemistry, Phase diagramsPhase DiagramPhase DiagramFinalAshcroft: 4-7Ashcroft: 4-7West: 5West: 7,8West: 11, 12Callister 9Chem 253, UC, BerkeleyMaterials Chemistry II11).Free Electron Model12).Nearly free electron model, Fermi surface,Effective Mass, Exciton.Fermi’s Golden Rule, Optical TransitionQuantum well/wire/dotGersten, 7,11Tight Binding ModelHoffmann,Burdett 1-3MO TheoryTight Binding ModelTight Binding ModelFinal13).14).15).16).17).18).Ashcroft/Mermin: 1-3, 8-10,Kittel: 6-93
Chem 253, UC, BerkeleySolid State and Materials ChemistrySynthesis, structure,properties, applications of solid materialsInorganic, organic, biologicalChem 253, UC, BerkeleyMaterials by DesignTheoretician: predict structure/composition withparticular propertiesChemist/Physicist: Make/measure/characterizeMaterials Genome Initiativehttp://www.whitehouse.gov/mgi4
Chem 253, UC, BerkeleyCharacterization Techniques:Organic/Organometallic: NMR, IR, crystallographySolid state:X-ray powder diffraction,Electron microscopyPhysical property characterizationChem 253, UC, BerkeleySuperconductivity1908, Kammerlingh-Onnes experiments on liquid He ( a few ml)Hg resistance: 0.08 ohm @ 5K to 0.000003 ohm @ 4.2 K1986, J. G. Bednorz, K. H. Muller (IBM)La-Ba-Cu-OOxide: Tc 35 K1997 Nobel prize in PhysicsFrench Group (B. Raveau)5
Chem 253, UC, BerkeleyBucky Ball; fullerene1996 Nobel prize in chemistryC60J.R. Heath, S.C. O'Brien, H.W. Kroto,R.F. Curl, R.E. Smalley,Nature 318 , 162, (1985)Chem 253, UC, BerkeleyConducting Polymer2000 Alan Heeger,Alan G. MacDiarmid,Hideki ShirakawaNobel Prize in Chemistry6
Chem 253, UC, BerkeleyGrapheneThe Nobel Prize in Physics 2010Andre Geim, Konstantin NovoselovChem 253, UC, BerkeleyMaterials Chemistry is the foundation for thefield ofNanoscience and technology.7
Chem 253, UC, BerkeleyEnergy ResearchChem 253, UC, BerkeleyCrystal StructureReading: Ashcroft 4-78
Chem 253, UC, BerkeleyCrystal StructureReading: Ashcroft 4-7Ideal Crystal: Contain periodical array of atoms/ionsRepresented by a simple lattice of pointsA group of atoms attached to each lattice pointsBasisLATTICE An infinite array of points in space, in which each pointhas identical surroundings to all others.CRYSTAL STRUCTURE The periodic arrangement of atoms in thecrystal.It can be described by associating with each lattice point a group ofatoms called the MOTIF (BASIS)Chem 253, UC, BerkeleyTranslationalvector{R n1 a1 n2 a2 n3 a3} in 3D9
Chem 253, UC, BerkeleyUNIT CELL The smallest component of the crystal, which whenstacked together with pure translational repetition reproduces thewhole crystalPrimitive Cell: simplest cell, contain one lattice pointNot necessary have the crystal symmetryChem 253, UC, BerkeleyWhite and black birdsby the artist, M. C. EscherUnit Cells?10
Chem 253, UC, BerkeleyConventional cell vs. Primitive CellReflecting the symmetryDifferent BasisChem 253, UC, BerkeleyBravais Lattice: an infinite array of discrete points with anarrangement and orientation that appears exactly the samefrom whichever of the points the array is viewed.PPNP5 Bravais Lattice in 2D11
Chem 253, UC, BerkeleySquarea b 90Rectangulara b 90CenteredRectangularHexagonala b 90a b 120Obliquea b 905 Bravais Lattice in 2DChem 253, UC, BerkeleyTranslationalvector12
Chem 253, UC, Berkeley3D: 14 Bravais Lattice, 7 Crystal SystemNameTriclinicNumber of Bravais lattices1 (P)Monoclinic2 (P, C)Orthorhombic4 (P, F, I, A)TetragonalCubic2 (P, I)3 (P, F, I)Trigonal1 (P)Hexagonal1 (P)Conditionsa1 a2 a3 a1 a2 a3 90 a1 a2 a3 90 a1 a2 a3 90 a1 a2 a3 90 a1 a2 a3 120 90 a1 a2 a3 90 120 Chem253, UC,BerkeleyAllowedrotationaxis:1, 2, 3, 4, 6NOT 5, 6Quasicrystal: AlFeCuShechtman, D., Blech, I., Gratias, D. & Cahn, J. W.Phys. Rev. Lett. 53, 1951-1953 (1984).The Nobel Prize in Chemistry 2011Dan Shechtman13
Chem 253, UC, BerkeleyCubic:four 3-fold three 4-foldChem 253, UC, BerkeleyUnit cell symmetries - cubic4 fold rotationaxes(passing through pairsof opposite facecenters, parallel to cellaxes)TOTAL 3 14
Chem 253, UC, BerkeleyUnit cell symmetries - cubic 4 fold rotation axesTOTAL 33-fold rotation axes(passing through cubebody diagonals)TOTAL 4Chem 253, UC, BerkeleyFCC LatticeCopper metal isface-centeredcubicIdentical atoms atcorners and at facecentersLattice type Falso Ag, Au, Al, Ni.15
Chem 253, UC, BerkeleyBCC Lattice -Iron is bodycentered cubicIdentical atoms atcorners and body center(nothing at face centers)Lattice type IAlso Nb, Ta, Ba, Mo.Chem 253, UC, BerkeleySimple Cubic LatticeCaesium Chloride(CsCl) is primitivecubicDifferent atoms atcorners and bodycenter. NOT bodycentered, therefore.Lattice type PAlso CuZn, CsBr,LiAg16
Chem 253, UC, BerkeleyFCC LatticesSodium Chloride (NaCl) Na is much smaller thanCsFace Centered CubicRocksalt structureLattice type FAlso NaF, KBr, MgO .Chem 253, UC, BerkeleyNature353, 147 - 149 (12 Sep 1991)17
Chem 253, UC, BerkeleyDiamond Structure: two sets of FCC LatticesChem 253, UC, BerkeleyTetragonal: P, IOne 4-fold axesWhy not F tetragonal?18
Chem 253, UC, BerkeleyExampleCaC2 - has a rocksalt-like structurebut with non-spherical carbidesCarbide ions arealigned parallel to c c a,b tetragonal symmetryChem 253, UC, BerkeleyOrthorhombic: P, I, F, CCF19
Chem 253, UC, BerkeleyAnother type of centeringSide centered unit cellNotation:A-centered if atom in bc planeB-centered if atom in ac planeC-centered if atom in abplaneChem 253, UC, BerkeleyTrigonal: P : 3-fold rotationa1 a2 a3 120 90 20
Chem 253, UC, BerkeleyHexagonala1 a2 a3 90 120 Chem 253, UC, BerkeleyMonoclinica1 a2 a3 Triclinica1 a2 a3 21
Chem 253, UC, BerkeleyLattice parameters:a, b, c; 7 Crystal SystemsChem 253, UC, BerkeleyThe choice of unit cell: reflect the crystal symmetry22
Chem 253, UC, BerkeleyUnit cell contentsCounting the number of atoms within the unit cellMany atoms are shared between unit cellsChem 253, UC, BerkeleyAtomscornerface centerbody centeredge centerlattice typePIFCShared Between:8 cells2 cells1 cell4 cellsEach atom counts:1/81/211/4cell contents1[ 8 x 1/8]2[ (8 x 1/8) (1 x 1)]4[ (8 x 1/8) (6 x 1/2)]2[ (8 x 1/8) (2 x 1/2)]23
Chem 253, UC, Berkeleye.g. NaClNa at corners: (8 1/8) 1Cl at edge centres (12 1/4) 3Na at face centres (6 1/2) 3Cl at body centre 1Unit cell contents are 4(Na Cl-)Chem 253, UC, BerkeleyFractional Coordinates(0,0,0)(0, ½, ½)(½, ½, 0)(½, 0, ½)24
Chem 253, UC, BerkeleyCs (0,0,0)Cl (½, ½, ½)Chem 253, UC, BerkeleyDensity Calculation nAVC N An: number of atoms/unit cellA: atomic massVC: volume of the unit cellNA: Avogadro’s number(6.023x1023 atoms/mole)Calculate the density of copper.RCu 0.128nm, Crystal structure: FCC, ACu 63.5 g/molen 4 atoms/cell, VC a 3 (2 R 2 ) 3 16 2 R 3(4)(63.5) 8.89 g / cm 38 323[16 2 (1.28 10 ) 6.023 10 ]8.94 g/cm3 in the literature25
Chem 253, UC, BerkeleyCrystallographic Directions And PlanesLattice DirectionsIndividual directions: [uvw]Symmetry-related directions: uvw Chem 253, UC, Berkeley26
Chem 253, UC, BerkeleyCrystallographic Directions And PlanesMiller Indices:1. Find the intercepts on the axes in terms of the latticeconstant a, b, c2. Take the reciprocals of these numbers, reduce to thethree integers having the same ratio(hkl)Set of symmetry-related planes: {hkl}Chem 253, UC, Berkeley(100)(200)(111)(110)27
Chem 253, UC, BerkeleyChem 253, UC, BerkeleyCrystallographic Directions And PlanesMiller-Bravais indices[uvtw], (hkil)t -(u v)i -(h k)28
Chem 253, UC, BerkeleyIn cubic system,[hkl] directionperpendicular to (hkl) planeChem 253, UC, BerkeleyLattice spacing1h2 k 2 l 2 2d hkla2For cubic system29
Chem 253, UC, BerkeleyChem 253, UC, BerkeleyCrystal Structure AnalysisX-ray diffractionElectron DiffractionNeutron DiffractionEssence of diffraction: Bragg Diffraction30
Chem 253, UC, BerkeleyInterference fringesLightConstructiveDestructiveChem 253, UC, BerkeleyBragg’s Lawn SQ QT d hkl sin d hkl sin 2d hkl sin For cubic system:d hkl ah2 k 2 l 2But not all planes have thediffraction !!!31
Chem 253, UC, BerkeleyX-Ray DiffractionE h hc / Mo: 35KeV 0.1-1.4ACu K 1.54 AChem 253, UC, BerkeleyX-Ray Diffraction32
Chem 253, UC, BerkeleyPowder diffractionX-Ray(200)Chem 253, UC, BerkeleyPowder diffraction33
Chem 253, UC, BerkeleyLaue DiffractionSinglecrystalChem 253, UC, BerkeleyElectron Diffraction34
Chem 253, UC, BerkeleyLayered CupratesThin film, growth orientedalong c axisExample: La2CuO22d sin n l)c 12.2 AChem 253, UC, Berkeleyc12.2 ACuO2LaOLaOCuO235
Chem 253, UC, BerkeleyExample: Ca0.5Sr0.5CuO22d sin n 2*thetad(hkl)12.76.96(001)263.42(002)Layered CupratesThin film, growth orientedalong c axis(00l)42.22.15(003)c 6.96 ÅChem 253, UC, Berkeleyc6.96 ÅSrCuO2Ca36
Cheethamand Day: Solid State Chemistry: Techniques (Oxford, 1987) Cheethamand Day: Solid State Chemistry: Compounds (Oxford, 1992) Cox: The Electronic Structure and Chemistry of Solids (Oxford, 1987) Wells: Structural Inorganic Chemistry (Clarendon Press, 1984) Wold and Dwight: Solid State Chemistry (Chapman Hall, 1993)
CHEM 350B Topics in Chemistry 7.5 454.95 CHEM 351 Chemicals Big and Small: Nano- 15 909.90 CHEM 352 Advanced Concepts in Chemistry 15 909.90 CHEM 352A Advanced Concepts in Chemistry 7.5 454.95 CHEM 352B Advanced Concepts in Chemistry 7.5 454.95 CHEM 360 Contemporary Green Chemistry 15 909.90 CHEM 380 Materials Chemistry 15 909.90
CHEM 31X. Chemical Principles 4 CHEM 33. Structure and Reactivity 4 CHEM 35. Organic Monofunctional Compounds 4 CHEM 36. Organic Chemistry Laboratory I 3 MATH 41, 42, 51. Calculus, Linear Equations 5 5 5 SECOND YEAR CHEM 130. Organic Chemistry Laboratory II 4 CHEM 131. Organic Polyfunctional Compounds y3 CHEM 134.
CHEM 110 Chemistry of the Living World 15 4,736.85 CHEM 120 Chemistry of Material World 15 4,736.85 CHEM 150 Concepts in Chemistry 15 4,736.85 CHEM 200 Special Topic 15 4,736.85 CHEM 251 Structure and Spectroscopy 15 4,736.85 CHEM 252 Properties and Analysis of Mat 15 4,736.85
WRF-Chem Version 3.9.1.1 User’s Guide Table of Contents 1.1 WRF-Chem Introduction3 1.2 WRF-Chem software 5 1.3 Possible applications of the current modeling system 5 1.4 The WRF-Chem modeling system overview 5 2.1 Software Installation Introduction 8 2.2 Building the WRF-Chem code 9 2.2.1 Getting the code 9
bonding and reactions) necessary for courses in elementary organic chemistry and physiological chemistry. Students may only receive credit toward graduation for one of the following: CHEM 10050; or CHEM 10060 and CHEM 10061; or CHEM 10970 and CHEM 10971.
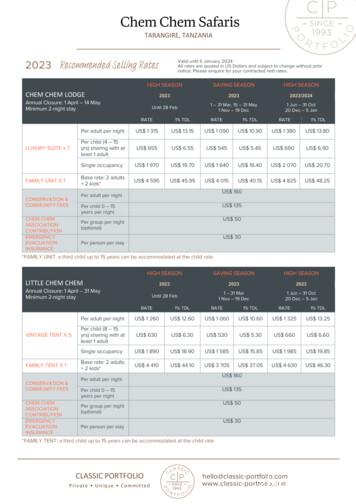
Chem Chem Safaris TARANGIRE, TANZANIA Updated: 9 November 2022 Page: 2 of 6 www.classic-portfolio.com CLASSIC PORTFOLIO Private Unique Committed hello@classic-portfolio.com www.classic-portfolio.com FOREST CHEM CHEM Annual Closure: 1 April - 31 May Minimum 2-night stay SAVING SEASON HIGH SEASON 2023 2023/2024 1 - 31 Mar 1 Nov - 19 Dec
RA 253 MA weld wire does not meet AWS classifications. Preheating and post-heating are not required for welding RA 253 MA. The chemistry of RA 253 MA welding wire and covered electrodes is balanced to have roughly a 4 to 12 Ferrite Number. This ferrite provides RA 253 MA weld fillers wi
1) Biology majors should take CHEM 104, CHEM 105 and CHEM 140 as early as possible in their B.Sc. program. (You should be taking CHEM 104 this semester, and should take at least one of CHEM 105/140 this coming January). 2) Students looking to take an elective Biology course are welcome in BIOL 100, but should