News Louisiana Board Of Pharmacy
NewsJanuary 2021LouisianaBoard of PharmacyPublished to promote compliance of pharmacy and drug law3388 Brentwood Drive Baton Rouge, LA 70809-1700 www.pharmacy.la.govBoard Elects Officers for Calendar Year2021 (21-01-655)During the November 18, 2020 meeting, the LouisianaBoard of Pharmacy members conducted their annual election of officers, with the following results: President – Carl W. Aron, from Monroe, LA, inDistrict 5 First Vice President – Marty R. McKay, fromWoodworth, LA, in District 8 Second Vice President – Jacqueline L. Hall, fromNew Orleans, LA, in District 2 Third Vice President – Rhonny K. Valentine, fromNatchitoches, LA, in District 4 Secretary – Richard M. Indovina, Jr, from RiverRidge, LA, in District 1Board Meeting Dates for Calendar Year2021 (21-01-656)The Board has announced the following tentativemeeting dates for calendar year 2021: February 23-25,May 25-27, August 17-19, and November 16-18. Based onconditions at the time due to the coronavirus disease 2019(COVID-19), the meetings may be physical or virtual.New Board Members (21-01-657)Governor John Bel Edwards appointed three newmembers to the Board in 2020: Pharmacist David A. Darce, who resides inBroussard, LA, and shares in the ownership of acommunity pharmacy in St Martinville, LA, wasappointed on June 1, 2020, to a six-year term endingJune 30, 2026. He replaces Mr Richard A. Soileau,who completed 12 years of service to the Board. Pharmacist Anthony G. Mercante, who residesin Ponchatoula, LA, and shares in the ownershipof a community pharmacy in the same town, wasappointed on June 1, 2020, to a six-year term endingJune 30, 2026. He replaces Mr Ronald E. Moore,who completed 12 years of service to the Board. Pharmacist J. Troy Menard, who resides in Covington, LA, and practices in a supervisory capacityfor a chain pharmacy, was appointed on October 2,2020, to serve the remainder of an unexpired termscheduled to expire on June 30, 2025. He replacesDr Sajal K. Roy, who served from November 2019to June 2020.The Board appreciates the years of dedicated servicefrom the previous members, and looks forward to workingwith the new members.Multiple Declarations of Emergency inEffect (21-01-658)Governor Edwards issued his initial declaration ofemergency for the COVID-19 public health emergencyon March 11, 2020; it has been renewed continuouslyand remains in effect at press time. The Board has issuedmultiple guidance documents, which have includedmultiple waivers and exemptions from certain rules. TheBoard has established a COVID-19 web page availableon the Board’s website now located under the State ofEmergency tab in the upper left corner of the home page.Governor Edwards issued his initial declaration for thesevere weather associated with Hurricane Laura on August21, 2020; it remains in effect until January 17. The Boardestablished a Hurricane Laura web page located under theState of Emergency tab. The posted guidance documentsand other resources include information on dispensingof emergency prescriptions, assessment of medicationintegrity in storm-damaged pharmacies, disposal ofcontaminated medical waste and hazardous waste, andreporting of theft or loss of controlled substances (CS).Decisions From November 2020 BoardMeeting (21-01-659)During the November 18, 2020 meeting, the membersmade several decisions affecting pharmacy practice,including: The members reviewed all 26 of the interimpolicies and waivers issued during the public healthcontinued on page 4LA Vol. 42, No. 3Page 1
National Pharmacy Compliance NewsJanuary 2021FOUNDATIONThe applicability of articles in the National Pharmacy Compliance News to aparticular state or jurisdiction can only be ascertained by examining the law ofsuch state or jurisdiction.DEA Publishes New Version of Pharmacist’sManualThe latest version of the Pharmacist’s Manual: AnInformational Outline of the Controlled Substances Act hasbeen released by Drug Enforcement Administration’s (DEA’s)Diversion Control Division. The guide is provided to assistpharmacists in understanding the Federal Controlled SubstancesAct and its regulations as they pertain to the pharmacyprofession. This edition has been updated to include informationon the Secure and Responsible Drug Disposal Act of 2010,the Comprehensive Addiction and Recovery Act of 2016, andthe SUPPORT for Patients and Communities Act of 2018, andreplaces all versions of the guidance previously issued by theagency. The new Pharmacist’s Manual can be accessed byvisiting the DEA website.Time to End VinCRIStine SyringeAdministrationThis column was preparedby the Institute for SafeMedication Practices (ISMP),an ECRI affiliate. Have youexperienced a medicationerror or close call? Report such incidents in confidence toISMP’s National Medication Errors Reporting Program online atwww.ismp.org or by email to ismpinfo@ismp.org to activate analert system that reaches manufacturers, the medical community,and Food and Drug Administration (FDA). To read more aboutthe risk reduction strategies that you can put into practice today, subscribe to the ISMP Medication Safety Alert! newsletters atwww.ismp.org.At the request of FDA, Pfizer has revised the prescribinginformation and product packaging for vinCRIStine sulfateinjection. Importantly, they have removed wording from thevinCRIStine package insert that described direct intravenous(IV) injection of vinCRIStine via a syringe. FDA recommendedthis revision at the request of ISMP, the National ComprehensiveCancer Network, and The Joint Commission.The WARNINGS section of the package insert now states, “Toreduce the potential for fatal medication errors due to incorrectroute of administration, vinCRIStine sulfate injection should bediluted in a flexible plastic container and prominently labeledas indicated ‘FOR INTRAVENOUS USE ONLY—FATALIF GIVEN BY OTHER ROUTES.’” More than 140 deathsare known to have occurred in the United States and globallydue to accidental intrathecal injection of the drug via syringe,often when it was mixed up with, or wrongly assumed it wassupposed to be given with, another drug meant for intrathecaladministration, such as methotrexate. No such cases havebeen reported with dilution of vinCRIStine in a minibag, dueto physical differences in the packaging and the need for anadministration set.Page 2NABPFNational Association of Boardsof Pharmacy FoundationUnfortunately, some practice sites are still using syringes toadminister IV vinCRIStine. Based on data collected in responseto the ISMP Medication Safety Self Assessment for High AlertMedications between September 2017 and March 2018 from 442US hospitals, nearly 20% of respondents still used syringes atleast part of the time, including 13% who always used syringesto administer IV vinCRIStine. Thus, the risk of accidentalintrathecal injection still exists in the US and globally.Dispensing vinCRIStine and other vinca alkaloids in aminibag of compatible solution, and not in a syringe, was amongthe very first ISMP Targeted Medication Safety Best Practices1for Hospitals, which were launched in 2014 . Then, in March2019, ISMP called on the FDA to eliminate all mention of syringe2administration from official vinCRIStine labeling.ISMP has frequently referred to wrong route administrationof vinCRIStine and vinca alkaloids as the “most serious of allmedication errors.” Patients experience tremendous pain and areoften aware of their impending death, which typically occurswithin days or weeks. There is no effective reversal once themistake is made. Even with the labeling change, there is nothingto stop health care practitioners from administering vinCRIStinevia syringe (except if they can only get the drug dispensed in aminibag). We hope that every hospital and health system willinvestigate exactly how vinCRIStine is being administered atany site that uses the drug. It is time to end the practice of syringeadministration by making it a requirement for all vinCRIStinedoses to be diluted in a minibag.References1. www.ismp.org/guidelines/best-practices-hospitals2. ngesvinca-alkaloidsWhat Pharmacists Need to Know AboutBiosimilar and Interchangeable BiologicalProductsThis column was prepared byFDA, an agency within the USDepartment of Health and Human Services, that protects thepublic health by assuring the safety, effectiveness, and securityof human and veterinary drugs, vaccines, and other biologicalproducts for human use, and medical devices. The agency alsois responsible for the safety and security of our nation’s foodsupply, cosmetics, dietary supplements, products that give offelectronic radiation, and for regulating tobacco products.Biological products are a diverse category of products andare generally large, complex molecules. These products maybe produced through biotechnology in a living system, such asa microorganism, plant cell, or animal cell, and are often moredifficult to characterize than small molecule drugs. There aremany types of biological products approved for use in the US,including therapeutic proteins (eg, filgrastim), monoclonal
National Pharmacy Compliance Newsantibodies (eg, adalimumab), and vaccines (eg, influenza andtetanus).Section 351(k) of the Public Health Service Act (PHS Act)provides an abbreviated licensure pathway for biologicalproducts shown to be biosimilar to or interchangeable with anFDA-licensed reference product, which can help provide moreaffordable treatment options for patients. For example, on March23, 2020, FDA-approved insulin products were transitioned toregulation as biological products under the PHS Act, whichmeans that transitioned insulin products are open to competitionfrom future biosimilars, including interchangeable biosimilars.Key Terms for Biosimilar and InterchangeableProducts Biosimilar Product: A biosimilar is a biological productthat is highly similar to and has no clinically meaningfuldifferences from an FDA-approved reference product. Interchangeable Product: An interchangeable product isa biosimilar that meets additional approval requirementsand may be substituted for the reference product withoutthe intervention of the prescribing health care provider. Reference Product: A reference product is the singlebiological product, already approved by FDA, againstwhich a proposed biosimilar or interchangeable productis compared.Are Biosimilars the Same as Generic Drugs?Biosimilars and generic drugs are versions of brand namedrugs and may offer more affordable treatment options topatients. Biosimilars and generics are approved through differentabbreviated pathways that avoid duplicating costly clinicaltrials. But biosimilars are not generics, and there are importantdifferences between them.For example, the manufacturer of a generic drug mustdemonstrate, among other things, that the generic contains thesame active ingredient as the brand name drug and that thegeneric is bioequivalent to the brand name drug. By contrast,biosimilar manufacturers must demonstrate that the biosimilaris highly similar to the reference product, except for minordifferences in clinically inactive components, and that thereare no clinically meaningful differences between the biosimilarand the reference product in terms of safety and effectiveness.Unlike generics, biosimilars are generally prescribed bybrand name by a health care provider, while interchangeables,like generics, may be substituted without the involvement ofthe prescribing health care provider, depending on state laws.What is the Purple Book?The Purple Book database contains information on FDAlicensed (approved) biological products regulated by the Centerfor Drug Evaluation and Research, including licensed biosimilarand interchangeable products, and their reference products. Italso contains information about all FDA-licensed allergenic,cellular, and gene therapy, hematologic, and vaccine productsregulated by the Center for Biologics Evaluation and Research .The Purple Book has simple and advanced search capabilitiesand some information that you can find includes the proprietary(brand) name and nonproprietary (proper) name of biologicalproducts, applicant (company), dosage form, product presentationJanuary 2021(eg, autoinjector, vial), route of administration, and strength. ThePurple Book also will display FDA-approved interchangeableproducts and note with which brand product each interchangeableproduct may be substituted.Are Therapeutic Equivalence Codes Assigned toBiological Products?FDA does not assign therapeutic equivalence codes tobiological products listed in the Purple Book like it doesfor small molecule drugs listed in the “Orange Book.” ThePurple Book provides information about whether a biologicalproduct has been determined by FDA to be biosimilar to orinterchangeable with a reference product.Can Interchangeable Products Be Substituted atthe Pharmacy?Many states have laws that address pharmacy-level substitution, including permitting substitution of interchangeableproducts, and the specific laws vary from state to state.There are currently no FDA-approved interchangeableproducts. Once there are, interchangeables, by definition, canbe expected to produce the same clinical result as the referenceproduct in any given patient.Biosimilar and interchangeable products meet FDA’s rigorousstandards for approval, and patients and health care providerscan be assured of the safety and effectiveness of these products,just as they would for the reference product.Where Can I Find Additional Resources? fda.gov/biosimilars purplebooksearch.fda.gov mation/deemed-be-license-provision-bpci-act fda.gov/media/135340/downloadFinal Insanitary Conditions at CompoundingFacilities Guidance Released by FDAContinuing efforts to protect patients from exposure to poorquality compounded drugs, FDA has published final guidancefor compounding facilities regarding insanitary conditions. Thefinal guidance, Insanitary Conditions at Compounding FacilitiesGuidance for Industry, provides recent examples of insanitaryconditions that FDA has observed at compounding facilitiesand details corrective actions that facilities should take whenthey identify these conditions. The guidance is intended to helpcompounders identify and prevent such issues at their facilities.While some compounders work hard to meet qualitystandards, FDA says its investigators continue to observe poorconditions that impact drug quality and that have the potentialto harm patients. These include the presence of dirt, mold,insects, trash, peeling paint, unclean exhaust vents, and dirtyhigh-efficiency particulate air filters.In response to the draft guidance, FDA states that it hasalso added recommendations for compounders to use riskmanagement tools to develop appropriate controls to preventinsanitary conditions at facilities. The guidance also addressesthe regulatory actions that FDA may take in response to theseconditions.Page 3
Louisiana Board of Pharmacy NewsJanuary 2021continued from page 1emergency as well as the declaration of emergencyfor Hurricane Laura. The members noted that someof the temporary waivers had already been convertedto permanent rules, some measures were no longernecessary, and some were still necessary. The Boardterminated some policies and extended others. Thestatus of all 26 interim policies may be verified underthe State of Emergency tab on the Board’s website.The Board also distributed this information by emailto all pharmacy licensees in early December. The members recalled an earlier decision in 2019 todelay enforcement of the federal standards on thehandling of hazardous drugs found in United StatesPharmacopeia Chapter 800 until January 1, 2021.They considered that pharmacies attempting tocomply with those standards have most likely beenimpacted by the current public health emergency.The members voted to further delay the enforcementof those standards to an undetermined date in thefuture beyond calendar year 2021. While the Boardhas opted to delay its enforcement, it is possiblepharmacies may have relationships with otherorganizations that have not delayed their expectationof compliance with those standards. The members took note of a temporary conflict inthe CS status of prescription cannabidiol. Whilethe federal government has removed that drug fromSchedule V of the federal list of CS, the LouisianaLegislature will not have the opportunity to makethat same decision until its next regular session laterthis year. In the interim, the Board voted to exerciseenforcement discretion and not take any disciplinary action against a pharmacy dispensing such prescriptions as non-CS. The Statement on DispensingPrescriptions for Cannabidiol resides on the Board’swebsite and was distributed by email to all pharmacylicensees in early December. Although some of the Board’s credentials currentlyexist only in virtual format, the Board voted toexpedite the transition of all of its credentials frompaper format to a virtual format, meaning no paperform will exist. All credentials are verifiable on theBoard’s website. Anyone needing a paper documentcan print the credential verification screen, whichincludes a notation that the Board’s website is a secureand primary source for credential verification, asauthentic as a direct inquiry to the Board. Staff willbegin transitioning credentials to virtual status. Itis possible some new credentials may be issued inpaper format with renewals converting to virtualformat.Disciplinary and Other LicensureActions (21-01-660)During its November 18, 2020 meeting, the Board tookaction on several items of business, including:Tamara Lynn Bourg (CPT.004979): Board grantedher request for reinstatement of the lapsed certificate,contingent upon satisfaction of the followingrequirements prior to November 18, 2022: (1) acquisitionof at least 250 hours of updated practical experienceunder the authority of a special work permit to berequested from the Board office; (2) receipt of a letterof competency from the pharmacist supervising theupdated practical experience; and (3) acquisition of atleast 10 hours of Accreditation Council for PharmacyEducation (ACPE)-accredited technician-specificcontinuing pharmacy education (CPE).Delana Rae Heltz (CPT.009588): Board granted herrequest for reinstatement of the lapsed certificate,contingent upon satisfaction of the followingrequirements prior to November 18, 2022: (1) acquisitionof at least 250 hours of updated practical experienceunder the authority of a special work permit to berequested from the Board office; (2) receipt of a letterof competency from the pharmacist supervising theupdated practical experience; and (3) acquisition of atleast 10 hours of ACPE-accredited technician-specificCPE.Jaime Lynn Blanchard (CPT.001387): Boardgranted her request for reinstatement of the lapsedcertificate, contingent upon satisfaction of thefollowing requirements prior to November 18, 2022:(1) acquisition of at least 250 hours of updatedpractical experience under the authority of a specialwork permit to be requested from the Board office; (2)receipt of a letter of competency from the pharmacistsupervising the updated practical experience; (3)acquisition of at least 10 hours of ACPE-accreditedtechnician-specific CPE; and (4) successful completionof a Board-approved pharmacy technician examination(Pharmacy Technician Certification Exam administeredby Pharmacy Technician Certification Board or, in thealternative, Exam for the Certification of PharmacyTechnicians administered by National HealthcareerAssociation).Louisiana CVS Pharmacy, LLC, dba CVS PharmacyNo. 4068 (Haughton, LA) (PHY.005771): For itsfailure to conduct a completed CS inventory followingthe discovery of a loss or theft of approximately 3,000tablets of alprazolam 1 mg on December 17, 2018, and forits failure to conduct a complete CS inventory followingthe discovery of a loss or theft of approximatel
3388 Brentwood Drive Baton Rouge, LA 70809-1700 www.pharmacy.la.gov January 2021 Published to promote compliance of pharmacy and drug law News Louisiana Board of Pharmacy Board Elects Officers for Calendar Year 2021 (21-01-655) During the November 18, 2020 meeting, the Louisiana Board of Pharmacy members conducted their annual elec -
April 2017. Published to promote compliance of pharmacy and drug law. News Louisiana Board of Pharmacy. Board Announces Personnel Changes in Compliance Division (17-04-540) The Louisiana Board of Pharmacy filled a long-standing vacancy in the chief compliance officer position by promoting
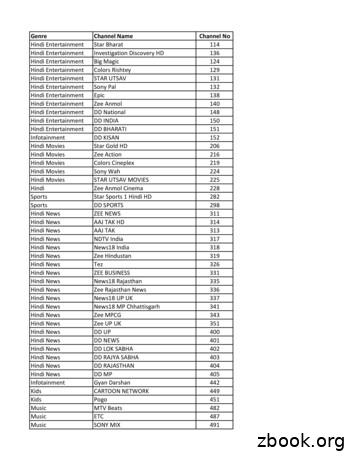
Hindi News NDTV India 317 Hindi News TV9 Bharatvarsh 320 Hindi News News Nation 321 Hindi News INDIA NEWS NEW 322 Hindi News R Bharat 323. Hindi News News World India 324 Hindi News News 24 325 Hindi News Surya Samachar 328 Hindi News Sahara Samay 330 Hindi News Sahara Samay Rajasthan 332 . Nor
Pharmacy Automation Supplies (PAS) Pharmacy Cure All, LLC (PCA) Pharmacy Development Services Pharmacy First Pharmacy Quality Solutions Pharmacy Technician Certification Board Pharmacy Times Pharmacy-Lite Packaging PharmPilot, Inc. PharmSaver LLC Pharmsource, LLC. Physician 360
Oct 14, 2009 · MM. CVS/pharmacy #9838 – Sun Valley NN. CVS/pharmacy #9840 – Reno OO. CVS/pharmacy #9841 – Reno PP. CVS/pharmacy #9842 – Carson City QQ. CVS/pharmacy #9843 – Fallon . Advanced Care Rx Pharmacy – Las Vegas CC. Prime Pharmacy – Henderson DD. Spring Valley Surgery Center
81 news nation news hindi 82 news 24 news hindi 83 ndtv india news hindi 84 khabar fast news hindi 85 khabrein abhi tak news hindi . 101 news x news english 102 cnn news english 103 bbc world news news english . 257 north east live news assamese 258 prag
Baton Rouge, Louisiana Ashley N. Freeman Lake Charles, Louisiana Samuel T. French Fayette, Mississippi Samantha G. Gahn Baton Rouge, Louisiana Landon P. Gauthier Gonzales, Louisiana John C. Ginart Chalmette, Louisiana Andres Gomez Lafayette, Louisiana . Taylor Alexander . Lake Charles, Louisiana
Louisiana Purchase PowerPoint Notes Answer Key Louisiana 1. Louisiana was the large area west of the Mississippi River. 2. 1762 - Louisiana was given to Spain after the French & Indian War. 3. 1800 - France took control of Louisiana New Orleans 4. What was the largest port in Louisiana? New Orleans 5. What were the American farmers worried .
Created Date: 9/14/2012 11:18:25 AM