April 2017 News Louisiana Board Of Pharmacy
NewsApril 2017LouisianaBoard of PharmacyPublished to promote compliance of pharmacy and drug law3388 Brentwood Drive Baton Rouge, LA 70809-1700 www.pharmacy.la.govBoard Announces Personnel Changes inCompliance Division (17-04-540)The Louisiana Board of Pharmacy filled a long-standingvacancy in the chief compliance officer position by promotingMr Benjamin Whaley from his former staff compliance officerposition, effective October 31, 2016. Mr Whaley brings nine yearsof experience in his staff role, preceded by 11 years of pharmacypractice experience.The Board is also pleased to announce the selection of a newstaff compliance officer. Ms Nicole L. Gross joined the Board’sstaff on March 6, 2017. She brings 20 years of pharmacy practiceexperience, most recently in hospital practice in the Shreveport,LA area. Ms Gross will be responsible for conducting inspectionsand investigations in the northwest corner of the state.The Board appreciates the professional courtesies extendedto all of our compliance officers. Congratulations, Benjamin, onyour promotion! Welcome to the family, Nicole!Renewal of Pharmacy Technician Certificates(17-04-541)The renewal cycle for pharmacy technicians will open onMay 1, 2017, and conclude on June 30. The Board no longer mailsrenewal application forms; instead, the Board office will mail arenewal reminder mailer just prior to May 1. The mailer will remind you of the three options you have to renew your certificate:1. Visit the Board’s website at www.pharmacy.la.gov andrenew your certificate online using a credit card;2. Visit the same website to download and print an applicationform, then complete and mail the application form with theappropriate fee using a check or money order; or3. Send a written request to the Board office (mail, fax, oremail) with your name, certificate number, and currentmailing address, requesting the Board to mail a paper application form to you.Any address changes received at the Board office after April14, 2017, will not be reflected on your renewal reminder mailer. Inthe event the postal service fails to deliver your renewal remindermailer by May 15, 2017, it then becomes your responsibility toobtain an application form or renew your certificate online. Certificates renewed online will be mailed within one or two businessdays; certificates renewed using paper application forms will bemailed within two to four weeks, depending on the volume ofpaper application forms received for processing.LA Vol. 38, No. 4The online renewal function of the website is programmed toactivate at 12:01 am on May 1 and to deactivate at midnight onJune 30, 2017. While the Board makes every effort to maintain thisonline convenience during the renewal cycle, the Board’s serviceprovider may experience weather-related or other unforeseentechnical difficulties from time to time. You have 60 days to renewyour certificate, and it is your choice as to when to complete thatduty. If you choose to wait until the last day and the website isnot available, then you will be responsible for the consequencesof your failure to renew your certificate in a timely manner.The Board does not waive late fees in that situation. Why take achance? Please do not wait until the last minute of the last day.All technician certificates expire on June 30 regardless of thedate of issue. You may not practice with an expired certificate.The fee for the timely renewal of an active certificate is 50. Forthe first 30 days past the expiration date, the renewal of an expiredcertificate will incur an additional 25 penalty fee, for a totalfee of 75. Applications received in the Board office more than30 days after the expiration date will incur an additional 200reinstatement fee, for a total fee of 275. Applications bearing apostal service mark of July 1 or later must be accompanied bythe additional fee(s) or the application package will be returnedto the sender unprocessed. If it is important to you to know if orwhen the Board receives your paper application form, the Boardsuggests you use the mail tracking service of your choice. Withalmost 7,000 certificates to be renewed, Board staff will not beable to respond to individual requests to confirm mail deliveries.Renewal of Other Credentials (17-04-542)In addition to the pharmacy technician cycle, the Board willbe renewing other credentials this spring and summer. Of thesecredentials, approximately: 500 automated medication system (AMS) registrationsexpire June 30; 475 emergency drug kit (EDK) permits expire June 30; 9,000 controlled dangerous substance (CDS) licenses forfacilities and practitioners expire between May 1 and July31; and 600 durable medical equipment (DME) permits expireAugust 31.The AMS, EDK, and CDS credentials must be renewed usingpaper application forms. The Board will mail those pre-printedapplication forms just prior to May 1, and timely renewals mustContinued on page 4Page 1
National PharmacyThe applicability of articles in the National Pharmacy Complianceby examining the law of sFOUNDATIONDEA Changes Registration Renewal ProcessAs of January 2017, Drug Enforcement Administration (DEA)will no longer send its second renewal notification by mail. Instead,an electronic reminder to renew will be sent to the email addressassociated with the DEA registration.In addition, DEA will retain its current policy and procedureswith respect to renewal and reinstatement of registration. Thepolicy is described below. If a renewal application is submitted in a timely mannerprior to expiration, the registrant may continue operations,authorized by the registration, beyond the expiration dateuntil final action is taken on the application. DEA allows the reinstatement of an expired registration forone calendar month after the expiration date. If the registrationis not renewed within that calendar month, an application fora new DEA registration will be required. Regardless of whether a registration is reinstated within thecalendar month after expiration, federal law prohibits thehandling of controlled substances or List 1 chemicals for anyperiod of time under an expired registration.Additional information is available on the DEA website at www.deadiversion.usdoj.gov/drugreg/index.html.ISMP Medication Safety Self Assessment forCommunity/Ambulatory PharmacyThis column was prepared by the Institutefor Safe Medication Practices (ISMP).ISMP is an independent nonprofit agencyand federally certified patient safety organization that analyzesmedication errors, near misses, and potentially hazardousconditions as reported by pharmacists and other practitioners.ISMP then makes appropriate contacts with companies andregulators, gathers expert opinion about prevention measures, andpublishes its recommendations. To read about the risk reductionstrategies that you can put into practice today, subscribe to ISMPMedication Safety Alert! Community/Ambulatory Care Editionby visiting www.ismp.org. ISMP provides legal protection andconfidentiality for submitted patient safety data and error reports.Help others by reporting actual and potential medication errors tothe ISMP National Medication Errors Reporting Program Reportonline at www.ismp.org. Email: ismpinfo@ismp.org.Pharmacists in community and ambulatory settings can nowaccess a newly revised tool that will help them review and improvetheir medication safety practices. The 2017 Institute for SafeMedication Practices (ISMP) Medication Safety Self Assessment for Community/Ambulatory Pharmacy is designed to help pharmacies evaluate their current systems, proactively identify opportunities for improvement, and track their efforts over time.An advisory panel of experts helped ISMP update items fromthe 2001 community/ambulatory self-assessment as well as additems to address new practices and processes, including thepharmacist’s evolving role in immunization administration. Newresearch findings about error prevention and emerging technologies previously not widely adopted are also covered.The self-assessment contains items that address the use ofmedications in the clinical setting, many of which are on thePage 2ISMP list of high-alert medications. Many of the items includedrepresent system improvements and safeguards that ISMP hasrecommended in response to analysis of medication errorsreported to the ISMP Medication Errors Reporting Program,problems identified during on-site consultations with health careorganizations, and guidelines in medical literature.The self-assessment is divided into 10 key elements that mostsignificantly influence safe medication use. Each element isdefined by one or more core characteristics of a safe pharmacysystem that further define a safe medication use system. Eachcore characteristic contains individual self-assessment items tohelp evaluate success with achieving each core characteristic.ISMP recommends that each pharmacy site convene its ownteam of staff members (ie, pharmacist(s), technician(s), and student pharmacist(s)) to complete this comprehensive assessmentand use the information as part of its ongoing safety and qualityimprovement efforts. An online form has been provided to helpparticipants organize and score their responses. Important: Theself-assessment should be completed in its entirety by staff andmanagers who work within the pharmacy, not by off-site managerson behalf of the pharmacy.When the self-assessment is completed, respondents can generate reports showing how their pharmacy answered each itemand how they scored on each as a percentage of the maximumpossible score. The pharmacy can then use its scores to identifyand prioritize opportunities for its safety plan of action.ISMP is not a regulatory or standards-setting organization. Assuch, the self-assessment characteristics represent ideal practicesand are not purported to represent a minimum standard of practice. Some of the self-assessment criteria represent innovativepractices and system enhancements that are not widely availablein pharmacies today. However, the value of these practices inreducing errors is grounded in expert analysis of medicationerrors, scientific research, or strong evidence of their ability toreduce errors.To view, download, and print the PDF of the assessment, whichincludes the introduction, instructions for use, self-assessmentitems, and definitions, visit DC Publishes Resource to Foster Use ofJCPP Pharmacists’ Patient Care ProcessA publication intended to encourage the use of the JointCommission of Pharmacy Practitioners (JCPP) Pharmacists’Patient Care Process was released by the Centers for DiseaseControl and Prevention’s (CDC’s) Division for Heart Diseaseand Stroke Prevention. In Using the Pharmacists’ Patient CareProcess to Manage High Blood Pressure: A Resource Guidefor Pharmacists, CDC calls on pharmacists and other healthcare providers to implement the Pharmacists’ Patient CareProcess model to reduce heart disease and stroke in the UnitedStates. Pharmacists can have a positive effect on populationhealth by providing patient care services and participating incollaborative practice agreements and continuing education(CE) programs, notes the CDC publication. The publicationis available at de.pdf.
Compliance Newse News to a particular state or jurisdiction can only be ascertainedsuch state or jurisdiction.The National Association of Boards of Pharmacy (NABP )is a member of JCPP and endorses the Pharmacists’ PatientCare Process. In its September 2015 newsletter (page 167),NABP discusses integrating the JCPP Pharmacists’ PatientCare Process to improve medication outcomes and promoteconsistency in patient care service delivery. Additional information about JCPP is available at https://jcpp.net.FDA Issues Final Guidance on RepackagingDrugs by Pharmacies and RegisteredOutsourcing FacilitiesIn January 2017, Food and Drug Administration (FDA)issued a final guidance for industry titled, “Repackaging ofCertain Human Drug Products by Pharmacies and OutsourcingFacilities.” This guidance describes the conditions underwhich FDA does not intend to take action for violations ofcertain provisions of the Federal Food, Drug, and CosmeticAct when a state-licensed pharmacy, a federal facility, or anoutsourcing facility repackages certain human drug products.The guidance is available at ic or written comments may be submitted at any timefor this final guidance following the instructions provided in theFederal Register, which can be found at ce.CriticalPoint Launches QP503A CertificationProgram for Sterile Compounding in 2017In 2017, CriticalPoint, LLC, launched its QP503A certification program for sterile compounding personnel. Specifically,CriticalPoint is offering the QP503A Certification and theQP503A Master Certification, which may be earned after obtaining the basic QP503A Certification. Participants will gain vitalknowledge and skills to successfully plan, develop, and operatea 503A pharmacy sterile compounding operation.The QP503A Certification involves a didactic program ofhome study, live training, and practicum activities accompanied by required objective personnel and cognitive testing. TheQP503A Master Certification requires participants to demonstrate their ability to apply their QP503A Certification training inactual work settings and produce measurable changes in sterilecompounding processes resulting in improved patient safety.Additional details about these programs and the certificationrequirements are available online at Point-QP503A-Certification.pdf.PTCB Suspends Implementation of Planned2020 Accredited Education Requirement forPharmacy TechniciansThe Pharmacy Technician Certification Board (PTCB) issuspending the implementation of the accredited education requirement for pharmacy technicians. In 2013, PTCB announcedthat the requirement would take effect in 2020, but PTCB has“determined that additional deliberation and research are neededNABPFNational Association of Boardsof Pharmacy Foundationto address stakeholder input, develop supporting policy, andconduct further study of technician roles,” said Larry Wagenknecht, BPharm, chair of the PTCB Board of Governors, andchief executive officer of the Michigan Pharmacists Association,in a news release. The role of pharmacy technicians is evolving,and PTCB is taking steps to support the pharmacy community.PTCB recently completed a job analysis study to collect dataon current roles and responsibilities of pharmacy techniciansacross all practice settings to update PTCB’s Pharmacy Technician Certification Exam and is in the process of developingadvanced certification programs. In addition, PTCB hostedan invitational conference in February 2017 where pharmacyleaders and stakeholders examined entry-level standards andprovided information to help determine future plans for implementing PTCB program changes.PTCB’s news release is available at www.ptcb.org in theNews Room section.ASOP Global Spreads Awareness AboutIllegal Online Drug Sellers and CounterfeitMedicationsAlliance for Safe Online Pharmacies (ASOP Global) partnered with several nonprofit organizations, including NABP,to launch a campaign to raise awareness of illegal online drugsellers and counterfeit medications. The campaign encouragesdialogue among health care providers and patients regardingwhere patients purchase their medications, especially if patients are buying them online.After offering the CE course “Internet Drug Sellers: WhatProviders Need to Know” to over 1,000 health care providers,ASOP Global found that less than 10% of providers reportedthey were “very aware” counterfeit prescription drugs are beingsold on the internet and only 1.4% said they regularly discussthe risks of illegal internet drug sellers with patients. ASOPGlobal Executive Director Libby Baney said, “After completing the course, however, there was a ten-fold increase in theexpected frequency in which providers planned to discuss therisks associated with buying prescription medicines onlinewith their patients and what they can do to avoid physicaland financial harm.”For more information about the campaign, visit www.BuySafeRx.pharmacy.New Interactive Map Tracks PharmacistVaccination LawsA new resource – an interactive 50-state map trackingpharmacist vaccination laws between 1990 and 2016 – waspublished by The Policy Surveillance Program, A LawAtlasProject. The map, which is available at n, explores laws that givepharmacists authority to administer vaccines and establishrequirements for third-party vaccination authorization, patientage restrictions, and specific vaccination practice requirements,such as training, reporting, record keeping, notification,malpractice insurance, and emergency exceptions. The PolicySurveillance Program is administered by Temple UniversityBeasley School of Law.Page 3
Continued from page 1be accomplished on or before the expiration date; penalties willapply to the renewal of expired credentials.The DME permits may be renewed either online or using paperapplication forms. The Board will mail the renewal remindermailer just prior to July 1, and timely renewals must be accomplished on or before August 31; penalties will apply to the renewalof expired credentials.Questions on Renewal Applications (17-04-543)Application forms for the renewal of pharmacy permits,pharmacist licenses, and pharmacy technician certificates containa series of questions requesting information concerning any legalissues or disciplinary actions taken against the applicant sincethe previous renewal. For each of those application forms, theinstructions request two documents in the event of an affirmativeanswer: a certified copy of the decision document from the courtor law enforcement agency, as well as the applicant’s personalletter of explanation.Two of these questions are commonly misinterpreted. Thefirst question requests whether the applicant has been arrested,charged, arraigned, indicted, or convicted, or whether the applicant has been issued a citation or summons, or whether the applicant has pled guilty, no contest, nolo contendere, or any similarplea, or whether the applicant has been sentenced or pardoned forany criminal offense, including misdemeanors and felonies, in anycourt in any local, state, or federal jurisdiction. The instructionsnote that the only offenses not required to be reported are minortraffic violations, such as speeding or parking tickets. The common mistake on this question is not reporting an arrest. It does notmatter whether the charges were not prosecuted, nor does it matterwhether any prior convictions have been expunged. The questionasks whether or not the action occurred; if it did, an affirmativereply is required. The applicant’s personal letter of explanationis the vehicle to inform the Board of the outcome of the incident.The second most common mistake relates to the question concerning civil or malpractice cases. The question asks whether theapplicant has been named in such a case. Simply being namedrequires an affirmative answer. The applicant’s personal letter ofexplanation is the vehicle to inform the Board of the outcome orstatus of the case.Affirmative replies to these questions do not automaticallyresult in disciplinary action by the Board. The Board considerseach case on its own merits and takes an action it deems as appropriate. For the majority of the disciplinary actions taken relatedto these matters, the issue that the applicant failed to disclose isnot the reason for the Board’s action; instead, the action is usuallytaken for the fail
April 2017. Published to promote compliance of pharmacy and drug law. News Louisiana Board of Pharmacy. Board Announces Personnel Changes in Compliance Division (17-04-540) The Louisiana Board of Pharmacy filled a long-standing vacancy in the chief compliance officer position by promoting
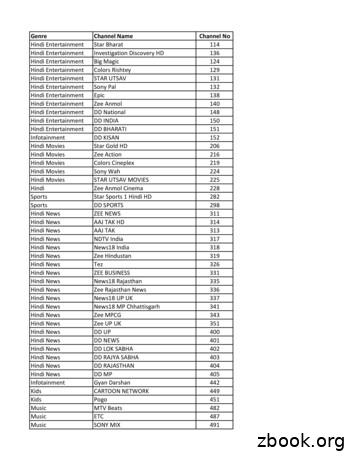
Hindi News NDTV India 317 Hindi News TV9 Bharatvarsh 320 Hindi News News Nation 321 Hindi News INDIA NEWS NEW 322 Hindi News R Bharat 323. Hindi News News World India 324 Hindi News News 24 325 Hindi News Surya Samachar 328 Hindi News Sahara Samay 330 Hindi News Sahara Samay Rajasthan 332 . Nor
81 news nation news hindi 82 news 24 news hindi 83 ndtv india news hindi 84 khabar fast news hindi 85 khabrein abhi tak news hindi . 101 news x news english 102 cnn news english 103 bbc world news news english . 257 north east live news assamese 258 prag
Baton Rouge, Louisiana Ashley N. Freeman Lake Charles, Louisiana Samuel T. French Fayette, Mississippi Samantha G. Gahn Baton Rouge, Louisiana Landon P. Gauthier Gonzales, Louisiana John C. Ginart Chalmette, Louisiana Andres Gomez Lafayette, Louisiana . Taylor Alexander . Lake Charles, Louisiana
Louisiana Purchase PowerPoint Notes Answer Key Louisiana 1. Louisiana was the large area west of the Mississippi River. 2. 1762 - Louisiana was given to Spain after the French & Indian War. 3. 1800 - France took control of Louisiana New Orleans 4. What was the largest port in Louisiana? New Orleans 5. What were the American farmers worried .
University Louisiana Lafayette: Upward Bound Math & Science (TRIO) Crystal Vallier cvallier@louisiana.edu Constance Broussard connie@louisiana.edu Shauna Landry Ahauna.landry@louisiana.edu Janice Nix Victorian jnix@louisiana.edu July 17-July18 (
Computer Science Nona Istre nona@louisiana.edu Informatics Dr. Hsiu-Yuen (Sonya) Hsu sonyahsu@louisiana.edu Environmental Science Dr. Durga Poudel ddpoudel@louisiana.edu Geology Dr. Tim Duex tduex@louisiana.edu Mathematics Dr. Ross Chiquet car4205@louisiana.edu Physics Dr. Andi Petculescu C00250270@louisiana.edu Department of Biology
3388 Brentwood Drive Baton Rouge, LA 70809-1700 www.pharmacy.la.gov January 2021 Published to promote compliance of pharmacy and drug law News Louisiana Board of Pharmacy Board Elects Officers for Calendar Year 2021 (21-01-655) During the November 18, 2020 meeting, the Louisiana Board of Pharmacy members conducted their annual elec -
Introduction In this unit we shall try to know about Aristotle and his life and works and also understand about the relationship between Criticism and Creativity. We shall see how criticism is valued like creative writings. We shall know the role and place given to 'the critic' in the field of literary criticism.