Essential Prescribing Information Device Description The-PDF Free Download
Do not withdraw Toujeo from the SoloStar and Max SoloStar single-patient-use prefilled pens with a syringe. References: 1. Toujeo Prescribing Information. 2. Basaglar Prescribing Information. 3. Lantus Prescribing Information. 4. Levemir Prescribing Information. 5. Tresiba Prescribing Information. 6. Toujeo Max SoloStar Instructions for Use.
Report of Use of the Prescribing Safety Assessment in MPharm Students and Preregistration Pharmacists 1. Background The Prescribing Safety Assessment1 was piloted in 2011/12 as a means of formatively assessing the prescribing abilities of final year medical students in response to concerns about prescribing competences in junior doctors.
Yoga for Healing: Why Western Doctors Are Now Prescribing Yoga Therapy 2/13/16, 2:26 PM prescribing-yoga-therapy/) PRESCRIBING-YOGA-THERAPY/) More Western Doctors Are Now Prescribing Yoga
Prescribing Safety Assessment Assessment Blueprint – August 2018 The Prescribing Safety Assessment has been developed by MSC Assessment and the British Pharmacological Society as a summative assessment of knowledge, judgement and skills related to prescribing medicines in the NHS. It is intended primarily for medical students at or
The Prescribing Safety Assessment 1 was piloted in 2011/12 as a means of formatively assessing the prescribing abilities of final year medical students in response to concerns about prescribing competences in junior doctors. This was extended to all medical schools in 2014 and in 2016
will go into effect on July 1, 2018. Select Florida Law on Controlled Substance Prescribing Prior to HB 21 There are numerous state and federal statutes and regulations that govern the prescribing of controlled substances. HB 21 amended several of the state statutes on controlled substance prescribing. These statutes are briefly discussed below.
DIR-330 A1 Device 6-18-2016 DIR-130 A1 Device 6-18-2016. DFE-690TXD A4 Device 6-8-2016 DFE-538TX F2 Device 6-8-2016 DFE-528TX E2 Device 6-8-2016 DXS-3250E A1 Device 5-31-2016 DXS-3250 A1 Device 5-31-2016 DXS-3227P A1 Device 5-31-2016 DXS-3227 A1 Device 5-31-2016 DEM-411T A1 Device 5-31-2016
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INBRIJA safely and effectively. See full prescribing information for INBRIJA. INBRIJA (levodopa inhalation powder), for oral inhalation use Initial U.S. Approval: 1970 _ INDICATIONS AND USAGE _
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ESBRIET safely and effectively. See full prescribing information for ESBRIET. ESBRIET. ESBRIET (pirfenidone) capsules and film-coated tablets, for oral use Initial U.S. Approval: 2014 discontinuations may be required.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use STIVARGA safely and effectively. See full prescribing information for STIVARGA. STIVARGA (regorafenib) tablets, for oral use Initial U.S. Approval: 2012 cardiac ischemia/infarction and re
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ABRAXANE safely and effectively. See full prescribing information for ABRAXANE. ABRAXANE for Injectable Suspension (paclitaxel protein-bound particles for injectable suspension) (albumi
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TRUSELTIQ safely and effectively. See full prescribing information for . If a dose of TRUSELTIQ is missed by 4 hours or if vomiting occurs, instruct patients to resume th
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include a increased systemic corticosteroid ll the information needed to use DULERA safely and effectively. See full prescribing information for DULERA. DULERA 100 mcg/5 mcg (mometasone furoate 100 mcg an
These highlights do not include all the information needed to use REMICADE safely and effectively. See full prescribing information for REMICADE. REMICADE (infliximab) for injection, for intravenous use Initial U.S. Approval: 1998 . WARNING: SERIOUS INFECTIONS and MALIGNANCY . See full prescribing information for complete boxed warning.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ONFI safely and effectively. See full prescribing information for ONFI. ONFI (clobazam) tablets, for oral use, CIV ONFI (clobazam) oral suspension, CIV Initial U.S. Approval: 2011 WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS;
1 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use CELLCEPT safely and effectively.See full prescribing information for CELLCEPT
Drug Drug Drug Drug P-gp P-gp PP-gp P -gp P gp Pradaxa [Prescribing information]. Ridgefield, CT: Boehringer-Ingelheim Pharmaceuticals, Inc; November 2015. Xarelto [Prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc; August 2016. Eliquis [Prescribing information].
The ASSI device can be configured to act as a master device or as a slave: Master device A master device is configured as device number 1. The master device connects to the Pitot tubes. Slave device A device configured with device number 2,3 or 4 is to be used in multi engine setups as a slave (see later chapter).
Prescribing Centre (now part of the National Institute for Health and Clinical Excellence (NICE)) publishes a range of materials to help you improve the safety and clinical and cost effectiveness of your prescribing. The electronic Medicines Compendium lists Summaries of Product Characteristics and Patient Information Leaflets.
A patient's ability to use an inhaler correctly is a crucial consideration when prescribing an inhaler device. Most patients can be taught to use a pressurised metered dose inhaler (pMDI), the least expensive and therefore, preferred device. Where a patient has difficulty using a pMDI, a spacer device or a breath-actuated MDI may be useful.
The Prescribing Safety Assessment (PSA) has been developed by MSC Assessment and the British Pharmacological Society as a summative assessment of knowledge, judgement and skills related to prescribing
2. The Prescribing Safety Assessment The PSA is an online assessment, delivered on a secure platform. The assessment is nationally developed but conducted locally by medical schools. The content of each item is relevant to the prescribing tasks expected of an F1 doctor, i.e. the questions refer to conditions and drugs likely to
The Prescribing Safety Assessment (PSA) has been jointly developed by the British Pharmacological Society and MSC Assessment. The PSA is intended to be a valid and reliable tool to demonstrate that medical graduates have achieved the core prescribing competencies outlined in the Outcome for graduates 2018 published by the GMC.
The Prescribing Safety Assessment (PSA) has been developed as an assessment of competence in relation to prescribing and supervising the use of medicines. This report describes the delivery of the PSA to all UK final-year medical students in 2016 (PSA2016). Methods. The PSA is a 2-hour online assessment comprising eight sections which cover various
mental health outcomes across the whole spectrum of disorders; and UÊ improving access to mainstream services and opportunities for people with long-term mental health problems. Social prescribing projects The most common examples of social prescribing are primary care-based
Medicines can do a lot of good but they also have the potential to cause harm. Medication errors are one of the most common causes of patient harm and prescribing accounts for a large proportion of medication errors. This evidence scan examines strategies to reduce prescribing er
BRIDGING THE GAP BETWEEN HEALTH AND HOUSING THROUGH SOCIAL PRESCRIBING Page 4 - 8 What is Social Prescribing Page 9 Typical Resident Journey Page 10- 11 The Benefits of Social Presc
The book is aimed not just at medical students, but also junior doctors, nursing staff, pharmacists and all those involved in prescribing and hospital care of patients. This book aims to empower you to excel at dealing with emergencies and handling complex prescribing scenarios. We wi
University of Derby Independent prescribing course reaccreditation event report - October 2020 3 Background The Independent prescribing (IP) programme at the University of Derby was initially accredited in 2003 and was last re-accredited in 2017 for a full period of three years; there were no conditions or recommendations.
Professional practice standards indicate an expectation that many elements of prescribing are undertaken by graduates within the studied professions. There is broad agreement between those with a vested interest in the assessment of prescribing (national regulatory boards, accrediting councils, professional
Antimicrobial Prescribing in Dentistry: Good Practice Guidelines ISBN: 978-1-8381964-2-4 e-ISBN: 978-1-8381964-3-1 Please cite this work as: Palmer, N. (Ed). Antimicrobial Prescribing in Dentistry: Good Practice Guidelines. 3rd Edition. London, UK: Faculty of General Dental Practice (UK) and Faculty of Dental Surgery; 2020.
its e-prescribing product, Rcopia . DrFirst also worked with the Drug Enforcement Administration (DEA) to test and implement its regulatory requirements for two-factor authentication and Electronic Prescribing of Controlled Substances (EPCS). Rcopia 4 EPCS Gold is an electronic prescription writing application and service which allows a prescriber
9100/F9100 SERIES CONCEALED VERTICAL ROD EXIT DEVICE 1135-26 I9100-6 8/06. 2 RHR LHR RHR LHR RHR LHR RHR LHR HANDING TYPICAL INSTALLATIONS SCREW CHART 9100 Series Device 9300 Series Device 9100 Series Device 9500 Series Device 9100 Series Device 9100 Series Device 9100
1 1 CZ:L96 2 PRESCRIBING INFORMATION 3 COMPAZINE 4 brand of 5 prochlorperazine 6 antiemetic antipsychotic tranquilizer 7 DESCRIPTION 8 Compazine (prochlorperazine) is a phenothiazine derivative, present in Compazine tablets and 9 Spansule sustained release capsules as the maleate. Its chemical name is 2-chloro-10-[3-(4-10 methyl-1-piperazinyl)propyl]
1.1 Production and Production Areas 3 1.1.1 Overview of the essential oils market and its composition 6 1.1.2 Identification of Products 6 2. HARVESTING OF ESSENTIAL OILS 7 2.1 Harvesting 7 2.2 Essential Oils Marketing Activities 7 3. EXPORTS VOLUMES 8 4. IMPORTS VOLUMES 19 5. USES OF ESSENTIAL OILS 25 6. QUALITY & MAINTANANCE OF ESSENTIAL OILS .
device on your compatible mobile device or computer. Select an option: Set up the device on your mobile device (Mobile Setup). Set up the device on your computer (Computer Setup). Mobile Setup Before you can pair your vívosmart device with your mobile device, your mobile
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling . Revised: 4/2021 . FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE . 2 DOSAGE AND ADMINISTRATION . 2.1 Dosing and Administration Information . 2.2 Dosage
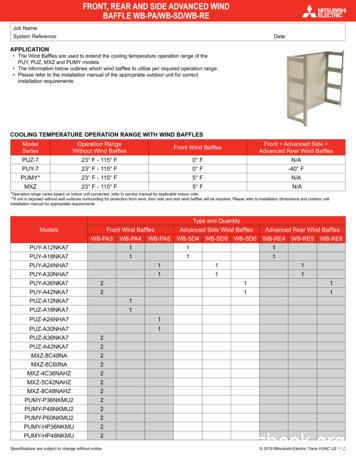
mitsubishi electric wind baffle - wb-pa3 mr. slim p-series pumy-60 submittal wb-pa3 description description c description d description e description f description g description proprietary and confidential the information contained in this drawing is the sole property
Essential Components of Behavior Intervention Plans Quick Guide Note to Practitioners: This quick guide is intended for use after prior training and extensive practice with the full Essential 10 Scoring Rubric, which includes more detailed information and examples. Additionally, the full Essential 10 provides information on which components of
see 17 for patient counseling information and medication guide. revised: 4/2020 . full prescribing information: contents* warning: post-injection delirium/sedation syndrome and increased mortality in elderly patients with dementia-related psychosis . 1 indications and usage . 1.