Microbiology Checklist
MasterEvery patientdeserves theGOLD STANDARD .Microbiology ChecklistCAP Accreditation ProgramCollege of American Pathologists325 Waukegan RoadNorthfield, IL 60093-2750www.cap.org04.21.2014
2 of 102Microbiology Checklist04.21.2014Disclaimer and Copyright NoticeOn-site inspections are performed with the edition of the Checklists mailed to a facility at the completionof the application or reapplication process, not necessarily those currently posted on the Web site. Thechecklists undergo regular revision and a new edition may be published after the inspection materials aresent.For questions about the use of the Checklists or Checklist interpretation, email accred@cap.org or call800-323-4040 or 847-832-7000 (international customers, use country code 001).The Checklists used for inspection by the College of American Pathologists' Accreditation Programs havebeen created by the CAP and are copyrighted works of the CAP. The CAP has authorized copying anduse of the checklists by CAP inspectors in conducting laboratory inspections for the Commission onLaboratory Accreditation and by laboratories that are preparing for such inspections. Except as permittedby section 107 of the Copyright Act, 17 U.S.C. sec. 107, any other use of the Checklists constitutesinfringement of the CAP's copyrights in the Checklists. The CAP will take appropriate legal action to protectthese copyrights.All Checklists are 2014. College of American Pathologists. All rights reserved.
3 of 102Microbiology Checklist04.21.2014Microbiology ChecklistTABLE OF CONTENTSSUMMARY OF CHANGES.5INTRODUCTION.8GENERAL MICROBIOLOGY.8PROFICIENCY TESTING.8QUALITY MANAGEMENT AND QUALITY CONTROL.9WAIVED TESTS.9GENERAL ISSUES.10SPECIMEN COLLECTION AND HANDLING.12REAGENTS - GENERAL.14REPORTING OF RESULTS.15INSTRUMENTS AND EQUIPMENT.16MATRIX-ASSISTED LASER DESORPTION IONIZATION TIME-OF-FLIGHT (MALDI-TOF) MASS Y.21MEDIA.21STAINS.23REAGENTS.24ANTIMICROBIAL SUSCEPTIBILITY TESTING, QC REQUIREMENTS, AND RESULTS REPORTING.26PROCEDURES AND TESTS.30RESPIRATORY SPECIMENS.31URINE SPECIMENS.32GENITAL SPECIMENS.32STOOL SPECIMENS.33CEREBROSPINAL & OTHER BODY FLUID SPECIMENS.34BLOOD CULTURES.35WOUND SPECIMENS.37GAS CHROMATOGRAPHY (GC) FOR MICROBIAL IDENTIFICATION.37LABORATORY SAFETY.40MYCOBACTERIOLOGY.40QUALITY CONTROL.40SPECIMEN HANDLING.41REPORTING OF RESULTS.41MEDIA.42CONTROLS AND STANDARDS.43PROCEDURES AND TESTS.44RAPID METHODS.44CONCENTRATION, INOCULATION, INCUBATION.45CULTURES.46DIFFERENTIAL BIOCHEMICAL PROCEDURES.46High Performance Liquid Chromatography (HPLC) for Microbial Identification.47LABORATORY SAFETY.50MYCOLOGY.52QUALITY CONTROL.52
4 of 102Microbiology Checklist04.21.2014MEDIA.52CONTROLS AND STANDARDS:.52PROCEDURES AND TESTS.54LABORATORY SAFETY.56PARASITOLOGY.58QUALITY CONTROL.58REAGENTS.59INSTRUMENTS AND EQUIPMENT.59PROCEDURES AND TESTS.60STOOLS FOR OVA AND PARASITES.60BLOOD FILMS FOR MALARIA AND OTHER PARASITES.61LABORATORY SAFETY.62VIROLOGY.64QUALITY CONTROL.64REAGENTS.64CONTROLS AND STANDARDS.67TESTS AND PROCEDURES.69LABORATORY SAFETY.70MOLECULAR MICROBIOLOGY.72GENERAL REQUIREMENTS.73QUALITY MANAGEMENT.73QUALITY CONTROL.74PROCEDURE MANUAL .77SPECIMEN HANDLING & PROCESSING.78RESULTS REPORTING.79REAGENTS .80PROCEDURES & TESTS.81INSTRUMENTS.82LABORATORY SAFETY.82PERSONNEL .83FDA CLEARED/APPROVED NON-AMPLIFICATION METHODS.83QUALITY CONTROL .84ASSAY VERIFICATION.85FDA CLEARED/APPROVED TARGET & SIGNAL AMPLIFICATION METHODS & SEQUENCING.86QUALITY CONTROL .86SEQUENCING.89ASSAY VALIDATION/VERIFICATION .90LABORATORY-DEVELOPED OR MODIFIED FDA CLEARED / APPROVED TESTS.91QUANTITATIVE ASSAYS: CALIBRATION & STANDARDS.91QUALITY CONTROL .95SEQUENCING .96TEST PROCEDURES.97ASSAY VALIDATION.98PERSONNEL - LABORATORY-DEVELOPED TESTS.101
5 of 102Microbiology Checklist04.21.2014ON-LINE CHECKLIST AVAILABILITYParticipants of the CAP accreditation programs may download the checklists from the CAP Web site (www.cap.org)by logging into e-LAB Solutions. They are available in different checklist types and formatting options, including: Master — contains ALL of the requirements and instructions available in PDF, Word/XML or Excel formatsCustom — customized based on the laboratory's activity (test) menu; available in PDF, Word/XML or ExcelformatsChanges Only — contains only those requirements with significant changes since the previous checklistedition in a track changes format to show the differences; in PDF version only. Requirements that havebeen moved or merged appear in a table at the end of the file.SUMMARY OF CHECKLIST EDITION CHANGESMicrobiology Checklist04/21/2014 EditionThe information below includes a listing of checklist requirements with significant changes in the current editionand previous edition of this checklist. The list is separated into three categories:1. New2. Revised: Modifications that may require a change in policy, procedure, or process for continued compliance;or A change to the Phase3. Deleted/Moved/Merged: Deleted Moved — Relocation of a requirement into a different checklist (requirements that have beenresequenced within the same checklist are not listed) Merged — The combining of similar requirementsNOTE: The listing of requirements below is from the Master version of the checklist. The customized checklistversion created for on-site inspections and self-evaluations may not list all of these requirements.NEW Checklist 4832Effective D Checklist MIC.14583MIC.14616Effective 2013
6 of 102Microbiology 9/201307/29/201307/29/2013DELETED/MOVED/MERGED Checklist 6500MIC.16525Effective 201404.21.2014
7 of 102Microbiology 201304.21.2014
8 of 102Microbiology Checklist04.21.2014INTRODUCTIONThis checklist is used in conjunction with the All Common and Laboratory General Checklists to inspect amicrobiology laboratory section or department.Certain requirements are different for waived versus nonwaived tests. Refer to the checklist headings and explanatorytext to determine applicability based on test complexity. The current list of tests waived under CLIA may be foundat cfClia/analyteswaived.cfm.Note for non-US laboratories: Checklist requirements apply to all laboratories unless a specific disclaimer ofexclusion is stated in the checklist.GENERAL MICROBIOLOGYRequirements in this section apply to ALL of the subsections in the microbiology laboratory (bacteriology,mycobacteriology, mycology, parasitology, molecular microbiology, and virology). When the microbiology departmentis inspected by a team, each member of the team must survey individual subsections for compliance withrequirements in this General Microbiology section. The team leader is then responsible for completing this sectionat the conclusion of the inspection.PROFICIENCY TESTINGInspector Instructions:MIC.00350 Are proficiency testing samples tested to the same level as clinical specimens? Select a representative clinical report of each culture type. Compare the extent ofreporting for the relevant proficiency testing sample.PT Extent of TestingPhase IIOrganisms in proficiency testing specimens are identified to the same level as those frompatient samples.NOTE: If the laboratory's proficiency testing reports include incomplete identifications (e.g. "Grampositive cocci" or "Mycobacterium species, not tuberculosis"), it must document that this matchesthe information produced by the laboratory's internal capabilities in patient reports. In other words,patient reports cannot be more specific than the identification level reporting in proficiency testing,unless the former contain more specific information provided by reference laboratories.
9 of 102Microbiology Checklist04.21.2014QUALITY MANAGEMENT AND QUALITY CONTROLInspector Instructions: Sampling of QC policies and proceduresSampling of QC recordsSampling of employee records of morphologic observation correlationWhat do you do if controls are out of range?How do you ensure consistency among personnel performing microscopic morphology?Select several occurrences in which QC is out of range and follow documentation todetermine if the steps taken follow the laboratory policy for corrective actionWAIVED TESTS**REVISED** 04/21/2014MIC.10060 Documented QC Results - Waived TestsPhase IIThe laboratory follows manufacturer instructions for quality control, and documents andreviews results for acceptability prior to reporting patient results.NOTE: Quality control must be performed according to manufacturer instructions. Testing personnelor supervisory staff must review quality control data on days when controls are run prior to reportingpatient results. The laboratory director or designee must review QC data at least monthly or morefrequently if specified in the laboratory QC policy.With respect to internal controls, acceptable control results must be documented, at a minimum,once per day of patient testing for each device.**Acceptable internal control results need not be documented, if (and only if) an unacceptableinstrument control automatically locks the instrument and prevents release of patient results.Evidence of Compliance: Written procedure consistent with manufacturer instructions for each waived test AND Records showing confirmation of acceptable QC resultsMIC.10070QC Corrective Action - Waived TestsPhase IIThere is documentation of corrective action when quality control results exceed theacceptable range.NOTE: The remaining requirements in this checklist on quality control do not apply to waived tests.
10 of 102Microbiology Checklist04.21.2014GENERAL ISSUESMIC.11015QC HandlingPhase IIControl specimens are tested in the same manner and by the same personnel as patientsamples.NOTE: QC specimens must be analyzed by personnel who routinely perform patient testing. Thisdoes not imply that each operator must perform QC daily, so long as each instrument and/or testsystem has QC performed at required frequencies, and all analysts participate in QC on a regularbasis. To the extent possible, all steps of the testing process must be controlled, recognizing thatpreanalytic and postanalytic variables may differ from those encountered with patients.Evidence of Compliance: Records reflecting that QC is run by the same personnel performing patient testingREFERENCES1) Department of Health and Human Services, Centers for Medicare and Medicaid Services. Clinical laboratory improvement amendmentsof 1988; final rule. Fed Register. 2003(Jan 24):7166 [42CFR493.1256(d)(8)].1256(f)]**REVISED** 04/21/2014MIC.11016 Commercial Product - QCPhase IIWhen using a commercial product, QC is performed according to the manufacturer'sinstructions or CAP Checklist requirements, whichever is more stringent.NOTE: This includes, but is not limited to, antimicrobial susceptibility testing/identification (AST/ID)systems.MIC.11017QC Confirmation of AcceptabilityPhase IIControl results are reviewed for acceptability before reporting patient results.Evidence of Compliance: Written policy/procedure stating that controls are reviewed and acceptable prior to reportingpatient results AND Evidence of corrective action taken when QC results are not acceptableREFERENCES1) Department of Health and Human Services, Centers for Medicare and Medicaid Services. Clinical laboratory improvement amendmentsof 1988; final rule. Fed Register. 2003(Jan 24)1992(Feb 28):7166 [42CFR493.1256(f)]MIC.11018QC Corrective ActionPhase IIThere is documentation of corrective action when control results exceed defined acceptabilitylimits.NOTE: Patient/client test results obtained in an analytically unacceptable test run or since the lastacceptable test run must be re-evaluated to determine if there is a significant clinical difference inpatient/client results. Re-evaluation may or may not include re-testing patient samples, dependingon the circumstances.Even if patient samples are no longer available, test results can be re-evaluated to search forevidence of an out-of-control condition that might have affected patient results.REFERENCES1) Department of Health and Human Services, Centers for Medicare and Medicaid Services. Clinical laboratory improvement amendmentsof 1988; final rule. Fed Register. 2003(Oct 1):1046[42CFR493.1282(b)(2)]
11 of 102Microbiology Checklist**REVISED** 04/21/2014MIC.11020 Monthly QC Review04.21.2014Phase IIQuality control data are reviewed and assessed at least monthly by the laboratory directoror designee.NOTE: The review of quality control data must be documented and include follow-up for outliers,trends, or omissions that were not previously addressed.The QC data for tests performed less frequently than once per month should be reviewed whenthe tests are performed.Evidence of Compliance: Records of QC review with documented follow-up for outliers, trends or omissionsMIC.11025Validation of AccuracyPhase IIIf the laboratory performs test procedures for which calibration and control materials arenot available, procedures have been established to validate the accuracy of patient testresults.REFERENCES1) Elder BL, et al. Verification and Validation of Procedures in the Clinical Microbiology Laboratory. Cumitech 31, February 1997. ASM2)MIC.11350Press; Washington DCSharp S and Clark R. Verification and Validation of Procedures in the Clinical Microbiology Laboratory. Cumitech 31a. 2009. ASM Pres:Washington, DCMorphologic Observation AssessmentPhase IIThe microbiology laboratory at least annually assesses morphologic observations amongpersonnel performing Gram, trichrome and other organism stains, to ensure consistency.NOTE: Suggested methods to accomplish this include:1. Circulation of organisms with defined staining characteristics, and/or2. Multi-headed microscopy, and/or3. Use of photomicrographs with referee and participant identifications (e.g. former CAPmicrobiology Surveys or other photomicrographs from teaching collections)4. Use of digital imagesEvidence of Compliance: Written procedure defining the method and criteria used for evaluation of consistency AND Employee records documenting morphology assessmentREFERENCES1) Flournoy DJ. Interpreting the sputum gram stain report. Lab Med. 1998;29:763-768**NEW** 04/21/2014MIC.11375 Taxonomy ChangesPhase IThe laboratory incorporates taxonomic changes that potentially affect the choice ofappropriate antimicrobials to report and/or the interpretative breakpoints to use.NOTE: The genus and/or species names of microorganisms may change as new methods areapplied to their taxonomy. This can impact the antimicrobials that should be reported for thatorganism. It may also change the breakpoints that should be used for interpreting susceptibility testresults. For example, Actinobacillus actinomycetemcomitans was moved to the genus Haemophilusin 1985 and then to the new genus Aggregatibacter in 2006.The antimicrobials differ for Haemophilusspecies (CLSI M100, Table 2E) versus Aggregatibacter species (CLSI M45, Table 7). The laboratoryshould have a procedure ensuring that clinically relevant taxonomic changes are incorporated intoreporting patient and proficiency testing results even when commercial identification systems havenot been updated.
12 of 102Microbiology Checklist04.21.2014REFERENCES1) Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently2)Isolated or Fastidious Bacteria; Approved Guideline—Second Edition. CLSI Document M45-A2 (ISBN 1-56238-732-4).Clinical andLaboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898 USA, 2010.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-FourthInformational Supplement. CLSI Document M100-S24 (ISBN 1-56238-898-3).Clinical and Laboratory Standards Institute, 940 WestValley Road, Suite 2500, Wayne, PA 19087-1898 USA, 2014.SPECIMEN COLLECTION AND HANDLINGCulture specimens are often collected by nurses or others outside the laboratory. An important aspect of qualitycontrol is the provision of adequate instructions to ensure proper collection and handling of specimens before theyare received by the laboratory.Inspector Instructions: MIC.13100Sampling of specimen collection and handling policies and proceduresSampling of requisitions for completenessSampling of specimen rejection records/log Sampling of microbiology specimens (transport media, timely delivery, labeling) What is your course of action when you receive unacceptable microbiology specimens?Specimen Acceptability CriteriaPhase IIThere are criteria for establishing specimen acceptability.NOTE: This could include important issues such as absence of gross external contamination,adequate specimen type/quantity, suitable preservation, prevention of dried swabs, and correct useof transport media when required.Evidence of Compliance: Records of rejected specimensMIC.13175Viral Culture SpecimensPhase ISpecimens for viral culture are collected appr
GENERAL MICROBIOLOGY Requirements in this section apply to ALL of the subsections in the microbiology laboratory (bacteriology, mycobacter iology , mycology , par asitology , molecular microbiology , and virology). When the microbiology depar tment is inspected by a team, each member of the t
An Introduction to Clinical Microbiology Susan M. Poutanen, MD, MPH, FRCPC . Objectives 1. To provide an introduction to a typical microbiology laboratory 2. To address specific microbiology laboratory test issues as they apply to public health. Department of Microbiology Who we are Shared microbiology service between TML (UHN & MDS) and MSH
Industrial microbiology Medical and pharmaceutical microbiology Rumen microbiology Space microbiology 1.2 Definitions Milk and milk products occupy a more significant role in the human food profiles. The study of microorganisms that are associated with milk and milk products in all aspects is defined as "Dairy Microbiology". 1.2 .
Manager Opening Checklist Line Check Prep Checklist Station Setup Bar Opening Checklist Closing Checklist Host Opening/Closing Checklist Multi‐unit Inspections Checklist Periodic Maintenance Checklist Permits & License Review Staff Reviews/Evaluations
General Microbiology Manual _ Abdelraouf A. Elmanama Ph. D Microbiology 7 Introduction Welcome to the microbiology laboratory. The goal of the laboratory is to expose students to the wide variety of lives in the microbial world. Although the study of microbiology includes
Title: Clinical Microbiology Users Handbook QP Ref: LH-MIC-GEN-G-001v1 Author: Jennifer Challoner & Alex Duggan Authorised by: Microbiology Specialty board Created Date:23rd April 2020 Disposal date: 22nd April 2050 Page 1 of 75 9693 Microbiology Laboratory Handbook Microbiology Laboratory North Tyneside General Hospital Rake Lane North Shields Tyne & Wear NE29 8NH This SOP supersedes all .
Microbiology H Core 4 3 30 70 100 4 MBH- 204 Food Microbiology H Core 4 3 30 70 100 4 MBS- 205 Bioinformatics S Core 2 2 15 35 50 2 Practical MBP- 206 Microbial Genetics, Molecular Biology Pract 4 4 30 70 100 4 MBP- 207 Environmental Microbiology and Food Microbiology Pract 4 4 30 70 100 4
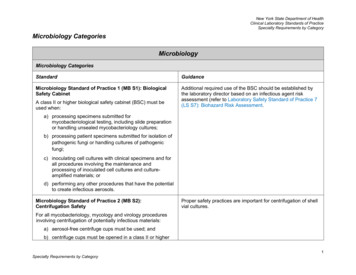
Microbiology Categories. Microbiology . Microbiology Categories Standard . Guidance; Microbiology Standard of Practice 1 (MB S1): Biological . Additional required use of the BSC should be established by the laboratory director
American Chiropractic Board of Radiology Heather Miley, MS, DC, DACBR Examination Coordinator PO Box 8502 Madison WI 53708-8502 Phone: (920) 946-6909 E-mail: exam-coordinator@acbr.org CURRENT ACBR BOARD MEMBERS Tawnia Adams, DC, DACBR President E-mail: president@acbr.org Christopher Smoley, DC, DACBR Secretary E-mail: secretary@acbr.org Alisha Russ, DC, DACBR Member-at-Large E-mail: aruss@acbr .