URGENT FIELD SAFETY NOTICE Fabian HFO And Fabian NCPAP . - Moph
Ventilation Beyond LimitsURGENT FIELD SAFETY NOTICEfabian HFO and fabian nCPAP evolution Volume Guarantee functionfor Field Safety Corrective Action FSCA-20-0015 October, 2020FSN Ref: FSCA-20-001-FSNAttention: Users of the fabian HFO and fabian nCPAP evolution ventilatorsDear Customer,The purpose of this communication is to inform you of a product Field Safety Corrective Action (FSCA)initiated by Acutronic Medical Systems AG (hereafter “Acutronic”), as part of Vyaire Medical, involving thefollowing fabian HFO and fabian nCPAP evolution ventilators.Details of affected devicesAffected software versions of fabian HFO and fabian nCPAP evolutionDeviceREF No.Description111001111001.01fabian HFONeonatal and pediatric ventilator112001113001fabian nCPAP evolution122001Software Version(s)5.0.x (with VG function)5.1.x (with VG function)Neonatal and pediatric ventilatorDescription of the problemMalfunction of VG function for fabian HFO and fabian nCPAP evolutionAcutronic has received reports of patient desaturation related to the following malfunction.The fabian HFOand fabian nCPAP evolution ventilators with the above-mentioned software (SW) versions can experiencea malfunction associated with over-delivery of peak-inspiratory pressure with delayed or absent alarmduring use of the Volume Guarantee (VG) function. This malfunction is associated with the breath-to-breathalgorithm and causes a temporary elevation of peak-inspiratory pressure (PIP) above the set Pmax for nolonger than 80ms which potentially leads to an increased risk of lung injury, hypoxia, barotrauma, andchanges to intrathoracic pressure.ACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h1/2
Ventilation Beyond LimitsActions to be taken by the users Make sure that the content of the complete FSCA package, including this FSN, is forwardedimmediately to any potential user of the fabian HFO and fabian nCPAP evolution ventilators usingthe Volume Guarantee (VG) function. Check receipt of FSCA package, containing this FSN, User Work Instructions TS-AA-027e, fabianInstruction (FI) Card, FSCA Response Form. In case affected devices are transferred to another location or organization make sure the completeFSCA package is forwarded to the respective users accordingly. Make sure that all devices affected are identified according to the User Work Instruction (TS-AA027e.) For identified devices, print out the fabian Instruction (FI) Card, make sure that it is available to allpotential users, keep it together with the Instructions for Use (IfU) and retain it until further notice. All users of the fabian HFO and fabian nCPAP evolution ventilators shall read and take intoconsideration the immediate mitigative actions below:Discontinue use of, and / or do not activate and use the optional VolumeGuarantee function with the affected fabian HFO and nCPAP evolution devices,until the Software update addressing the Volume Guarantee malfunction isinstalled.This malfunction does not affect the general use of the ventilators and only impactsthe use of the Volume Guarantee function. Other functions of the ventilators are notaffected. The ventilators may continue to be used for all ventilation modes of therapy,without using the Volume Guarantee function.For infants with severe lung disease, alternative forms of lung protective ventilationmay be considered.The ongoing FSCA-18-004 related to the software version 5.1.0 is a mandatorysoftware update to correct identified issues affecting previous software versions. Anydevices that have not yet been updated to software version 5.1.0 must still be updatedwith the FSCA-18-004 software. However, the VG function on these updated devices Fully complete and return the signed FSCA Response Form to Acutronic directly as per theinstructions on the form.Contact InformationFor questions, concerns or any events that reasonably suggest being related to the subject of this FSCAor to related Forms, please email GMB-AMS-FSCAresponsecentre@vyaire.comThe undersigned confirms that this notice has been provided to the appropriate Regulatory Agencies.Sincerely,Abir RoyQA Manager, Post Market Surveillance / FSCA CoordinatorFabrik im SchiffliCH-8816 HirzelSwitzerlandPhone: 41 447297098Email: abir.roy@vyaire.comACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h2/2
Ventilation Beyond LimitsField Safety Corrective Action Response FormFSCA-20-001Details of affected parts and products:Affected ProductsModelREF No.fabian HFOfabian HFO Lightfabian HFOifabian HFOi Lightfabian nCPAP evolutionSoftware Version(s)112001111001113001111001.011220015.0.x (with VG function)5.1.x (with VG function)Refer to the User Work Instruction (TS-AA-027e) and the fabian Information (FI) Card forfurther information about how to identify affected devices and instructions to follow for thisFSCA.User DeclarationNumber of affected devices (total)FOR EACH AFFECTED DEVICE PLEASE PROVIDE (if necessary, attach extra page)Device Serial NumberSoftware VersionPlease verify the following by checking the box below.I have received the FSCA package, including the Field Safety Notice (FSCA-20-001-FSN),the FSCA Response Form, User Work Instruction TS-AA-027e, and the fabian Instruction(FI) Card), and understood the content and will follow and implement the instructionsaccordingly.I confirm that all users of the affected devices were and will be informed about the FSCAimmediately and the FSCA package provided.I have identified all affected devices and have entered them on or attached them to thisresponse form.I have printed out the fabian Instruction (FI) Card for affected devices and ensured it is orwill be made available to all potential users, is kept together with the Instructions for Use,and will be retained until further notice.ACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h1/2
Ventilation Beyond LimitsI confirm that affected devices were transferred to another location/organization and thecomplete FSCA package was forwarded to the respective users accordingly. List contact details of recipients.User DetailsContact person (name)Hospital (address)Countryemail addressDateSignaturePLEASE SEND THIS RESPONSE FORM TO THE FOLLOWING ADDRESS:Contact InformationFor questions, concerns or any events that reasonably suggest being related to the subject of thisFSCA or to related Forms, please email GMB-AMS-FSCAresponsecentre@vyaire.comMr. Abir RoyQA Manager, Post Market Surveillance / FSCA CoordinatorFabrik im SchiffliCH-8816 HirzelSwitzerlandPhone: 41 447297098Email: abir.roy@vyaire.comACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h2/2
Ventilation Beyond LimitsFI Card-20-001.DEHFO / nCPAP evolution - FSCA-20-001Funktion „Volumengarantie“ (VG) nicht verwendenBeenden Sie die Verwendung der optionalen Funktion „Volumengarantie“ mit einembetroffenen Beatmungsgerät fabian HFO und nCPAP evolution und/oder aktivieren undverwenden Sie diese Funktion nicht, bis das Software-Update installiert wurde, das dieFehlfunktion der Volumengarantie behebt.Diese Fehlfunktion beeinflusst nicht die generelle Verwendbarkeit der Beatmungsgeräte und betrifft nur dieAnwendung der Funktion „Volumengarantie“. Die anderen Funktionen der Beatmungsgeräte sind nichtbeeinträchtigt. Die Beatmungsgeräte können weiterhin in allen Beatmungs-Modi genutzt werden, ohne dieFunktion „Volumengarantie“ anzuwenden. Bei Säuglingen mit schwerer Lungenerkrankung sollten andereFormen der lungenschonenden Beatmung erwogen werden.fabian HFO: Funktion „Volumengarantie“ (VG) abschaltenWenn die in der unten stehenden Abbildung gezeigteSchaltfläche grün ist, ist die VG-Funktion aktiviert.Bitte deaktivieren Sie die Funktion, indem Sie auf diegezeigte Schaltfläche drücken.Wenn die in der unten stehenden Abbildung gezeigteSchaltfläche nicht grün ist, ist die VG-Funktiondeaktiviert. Aktivieren Sie die Funktion nicht.fabian nCPAP evolution: Funktion „Volumengarantie“ (VG) abschaltenWenn die in der unten stehenden Abbildung gezeigteSchaltfläche grün ist, ist die VG-Funktion aktiviert.Bitte deaktivieren Sie die Funktion, indem Sie auf diegezeigte Schaltfläche drücken.Wenn die in der unten stehenden Abbildung gezeigteSchaltfläche nicht grün ist, ist die VG-Funktiondeaktiviert. Aktivieren Sie die Funktion nicht.HINWEIS: Die VG-Fehlfunktion wird durch die Installation eines Software-Upgrades (derzeit in Entwicklung) auf Ihrem Gerätbehoben. Die VG-Funktion kann danach verwendet werden. Sobald die neue Software verfügbar ist, wird IhrAcutronic/Vyaire-Vertriebspartner Sie informieren. Bezüglich der Installation des Software-Updates wenden Sie sich bitte anIhren Acutronic/Vyaire-Vertriebspartner, einen autorisierten technischen Servicetechniker oder Ihren Handelsvertreter.Rev. 1.0File: fabian instruction FI Card-20-001.DE ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h
Ventilation Beyond LimitsFI Card-20-001.ENHFO / nCPAP evolution - FSCA-20-001Do not use the Volume Guarantee (VG) functionDiscontinue use of, and / or do not activate and use the optional Volume Guarantee functionwith the affected fabian HFO and nCPAP evolution devices, until the Software updateaddressing the Volume Guarantee malfunction is installed.This malfunction does not affect the general use of the ventilators and only impacts the use of the VolumeGuarantee function. Other functions of the ventilators are not affected. The ventilators may continue to be usedfor all ventilation modes of therapy, without using the Volume Guarantee function.For infants with severe lung disease, alternative forms of lung protective ventilation may be considered.fabian HFO: switch off Volume Guarantee (VG) functionWhen the softkey indicated below is green, the VGfunction is activated. Please deactivate it by pushingthe indicated softkey below.If the indicated softkey below is not green, the VGfunction is deactivated. Do not activate.fabian nCPAP evolution: switch off Volume Guarantee (VG) functionWhen the softkey indicated below is green, the VGfunction is activated. Please deactivate it by pushingthe indicated softkey.If this button is not green, the VG function isdeactivated. Do not activate.NOTE: The VG malfunction will be fixed by installing a software update (currently in development) on your device. The VGfunction may be used thereafter. Once the new software is available, your Vyaire Distribution partner will inform you via EndUser Release Note. Regarding the installation of the software update, please refer to your Acutronic/Vyaire Distributionpartner, authorized technical service engineer or your sales representative.Rev. 1.0File: fabian instruction FI Card-20-001.DE ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h
FSCA-20-001User Work InstructionVolume Guarantee functionfabian HFO and fabian nCPAP evolution
Ventilation Beyond LimitsTable of contents:1. How to identify affected devices . 31.1 How can you determine whether your devices are affected? . 31.2 How to check whether the VG function is available on your device . 41.2.1 fabian HFO: check whether the VG function is available on your device . 41.2.2 fabian nCPAP evolution: check whether the VG function is available on your device . 42. How to identify your device is affected or not . 53. What to do if your devices are affected . 10Document No: TS-AA-27 Volume Guarantee functionDate: 2020-10-5Unit: fabianOur Ref: FSCA-20-001Software:5.0.X, 5.1.XHardware: fabian HFO, fabian nCPAP evolutionDescription: FSCA-20-001 fabian HFO and fabian nCPAP evolution Volume Guarantee (VG)function Mandatory Where necessary As InformationRevision 1.0: CreationRev. 1.0File: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h2/10
Ventilation Beyond Limits1. How to identify affected devices (FSCA-20-001)1.1How can you determine whether your devices are affected?Turn on the device. On the main screen, press 2xpress the “Info” buttonCheck the Software version available on the fabian HFO or fabian nCPAP evolution devicee.g: affected software versionRev. 1.0e.g: not affected software versionFile: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h3/10
Ventilation Beyond Limits1.2How to check whether the VG function is available on your device1.2.1 fabian HFO: check whether the VG function is available on your device1. switch to IPPV mode2. press an the “SETTINGS” button1.2.23. check whether the Volume Guarantee (VG)button is available. If yes, the VG option isavailable.fabian nCPAP evolution: check whether the VG function is available on your device1. press the “Ventilation” button2. press 2x the “ ” button3. check whether the Vguarant field is available. Ifyes, the VG function is available4. If not, the VG option is not available.Rev. 1.0File: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h4/10
Ventilation Beyond Limits2. How to identify your device is affected or not1. fabian HFOfabian HFO: overview/identification FSCA-20-001 affected software version(s)ModelVG option installed?fabian HFORev. 1.0SW V5.0.XSW V5.1.XSW es--NoNo-Yes-NoNo--YesNoFile: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h5/10
Ventilation Beyond Limits2. fabian HFO Lightfabian HFO Light: overview/identification FSCA-20-001 affected software version(s)ModelVG option installed?fabian HFO LightRev. 1.0SW V5.0.XSW V5.1.XSW es--NoNo-Yes-NoNo--YesNoFile: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h6/10
Ventilation Beyond Limits3. fabian HFOifabian HFOi: overview/identification FSCA-20-001 affected software version(s)ModelVG option installed?fabian HFOiRev. 1.0SW V5.0.XSW V5.1.XSW es--NoNo-Yes-NoNo--YesNoFile: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h7/10
Ventilation Beyond Limits4. fabian HFOi LightSame front design as HFOi, but serial number starts with AL. for example: AL-XXXXXfabian HFOi Light: overview/identification FSCA-20-001 affected software version(s)ModelVG option installed?fabian HFOi LightRev. 1.0SW V5.0.XSW V5.1.XSW es--NoNo-Yes-NoNo--YesNoFile: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h8/10
Ventilation Beyond Limits5. fabian nCPAP evolutionfabian nCPAP evolution: overview/identification FSCA-20-001 affected software version(s)ModelVG option installed?fabian nCPAPevolutionRev. 1.0SW V5.0.XSW V5.1.XSW es--NoNo-Yes-NoNo--YesNoFile: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h9/10
Ventilation Beyond Limits3. What to do if your devices are affected1. Print out the fabian Instruction Card (FI Card-20-001) that was provided to you by theDistributor as part of the FSCA-20-001 package.The fabian Instruction Card describes how to determine if the Volume Guarantee function isactive and how to deactivate it. Keep this document together with the IFU of the affecteddevices.2. Make sure that all potential users of the affected devices read and understand the contentsof the fabian Instruction Card (FI Card-20-001).Following the instructions in the fabian Instruction Card (FI Card-20-001), discontinue use of, and/ or do not activate and use the optional Volume Guarantee function with the affected fabianHFO and nCPAP evolution devices, until the Software update addressing the Volume Guaranteemalfunction is installed.This malfunction does not affect the general use of the ventilators and only impacts the use of theVolume Guarantee function. Other functions of the ventilators are not affected. The ventilatorsmay continue to be used for all ventilation modes of therapy, without using the Volume Guaranteefunction.For infants with severe lung disease, alternative forms of lung protective ventilation may beconsidered.The ongoing FSCA-18-004 related to the software version 5.1.0 is a mandatory software update tocorrect identified issues affecting previous software versions. Any devices that have not yet beenupdated to software version 5.1.0 must still be updated with the FSCA-18-004 software. However,the VG function on these updated devices must not be used.NOTE: The VG malfunction will be fixed by installing a software upgrade (currently in development)on your device. The VG function may be used thereafter. Once the new software is available, yourAcutronic/Vyaire Distribution partner will inform you via End User Release Note. Regarding theinstallation of the software update, please refer to your Acutronic/Vyaire Distribution partner,authorized technical service engineer or your sales representative.Rev. 1.0File: User Work Instruction TS-AA-027 ENACUTRONIC Medical Systems AGFabrik im SchiffliCH-8816 Hirzel SwitzerlandPhone 41 44 729 70 80Fax 41 44 729 70 h10/10
Ventilation Beyond LimitsDistributor’s Field Safety Corrective Action Response FormFSCA-20-001Affected Products (according to FSN)Device ModelREF No.fabian HFOfabian HFO Lightfabian HFOifabian HFOi Lightfabian nCPAP evolution112001111001113001111001.01122001Software Versions5.0.x (with VG function)5.1.x (with VG function)Distributor Self-declarationNumber of affected devices on the marketNumber of affected devices in stockFOR EACH AFFECTED DEVICE PLEASE PROVIDE (if necessary, attach extra page)Device Serial NumberSoftware VersionUser facility name and addressMark applicable check boxes below.All affected devices and users were identified.The FSCA package, incl. the Field Safety Notice (FSCA-20-001-FSN),User Work Instructions (TS-AA-027e), fabian Instruction (FI) Card and FSCA ResponseForm has been distributed to all identified affected users.The FSCA package, incl. the Field Safety Notice (FSCA-20-001-FSN),User Work Instructions (TS-AA-027e), fabian Instruction (FI) Card and FSCA ResponseForm has not been distributed to all identified affected users. Provide rationale below:Target Response Rate set by Acutronic Medical Systems AG:100%2/21/2Dateiname:Filename:Distributor Response Form FSCA 20-001Copyright ACUTRONIC Medical Systems AG. Alle Rechte vorbehalten. Nur für internen Gebrauch / All rights reserved. For internal use only.Nach ausdrucken des Dokuments ist es nicht mehr unter Kontrolle / After printing the document it is no longer under control
Ventilation Beyond LimitsBy signing this form, I certify the following (mark applicable check boxes)I have read and understood the content of the Field Safety Notice (FSCA-20-001-FSN) andconfirm that I understand all instructions noted within the Field Safety Notice (FSN).All users were informed about this FSCA and the FSCA package provided.Users were informed via: Mail E-mail Other:I confirm that affected devices were transferred to another location/organization and thecomplete FSCA package was forwarded to those recipients. List contact details of recipientsI was not able to fulfil the requirements specified in the FSN. RationaleI do not have fabian ventilators in stock nor on the market.I am not a fabian ventilator distributor, therefore I am not affected by this FSCA and no furtheractions are required. RationaleDistributor (company name)Address of DistributorEmail addressTelephone numberName of person completing form(Please Print)Function of person completing formSignature of person completing formDateTHIS FORM MUST BE RETURNED TO THE FOLLOWING ADDRESS.ONLY FULLY COMPLETED AND SIGNED FORMS WILL BE ateiname:Filename:Distributor Response Form FSCA 20-001Copyright ACUTRONIC Medical Systems AG. Alle Rechte vorbehalten. Nur für internen Gebrauch / All rights reserved. For internal use only.Nach ausdrucken des Dokuments ist es nicht mehr unter Kontrolle / After printing the document it is no longer under control
ACUTRONIC Medical Systems AG Fabrik im Schiffli CH-8816 Hirzel Switzerland Phone 41 44 729 70 80 Fax 41 44 729 70 81 info@acutronic-medical.ch www.acutronic-medical.ch 1/2 . URGENT FIELD SAFETY NOTICE fabian HFO and fabian nCPAP evolution Volume Guarantee function
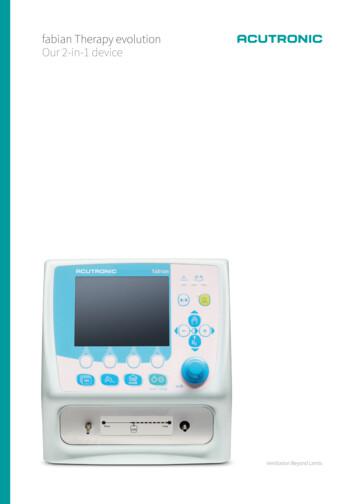
fabian nCPAP evolution our 3-in-1 device The fabian nCPAP evolution is a therapy system encased in a small yet powerful device. Ideal for NICU, PICU and transport applications. fabian Therapy evolution our 2-in-1 device The fabian Therapy evolution is the smallest but not the least powerful fabian family member. A true, complete
not the least powerful fabian family member. A true, complete and highly advanced non-invasive ventilator featuring all classic and new NIV modes. The fabian nCPAP evolution is a therapy system encased in a small yet powerful device. Ideal for NICU, PICU and transport applications. HFNC NIV CV HFO HFNC NIV CV fabian HFO Our 4-in-1 device.
not the least powerful fabian family member. A true, complete and highly advanced non-invasive ventilator featuring all classic and new NIV modes. The fabian nCPAP evolution is a therapy system encased in a small yet powerful device. Ideal for NICU, PICU and transport applications. HFNC NIV CV HFO HFNC NIV CV fabian HFO Our 4-in-1 device.
The fabian Therapy evolution is the smallest but not the least powerful fabian family member. A true, complete and highly advanced non-invasive ventilator featuring all classic and new NIV modes. The fabian nCPAP evolution is a therapy system encased in a small yet powerful device. Ideal for NICU, PICU and transport applications. HFNC NIV CV HFO
The fabian Therapy evolution is the smallest but not the least powerful fabian family member. A true, complete and highly advanced non-invasive ventilator featuring all classic and new NIV modes. The fabian nCPAP evolution is a therapy system encased in a small yet powerful device. Ideal for NICU, PICU and transport applications. HFNC NIV CV HFO
Feb 14, 2016 · 01 First Assist Urgent Care - Abingdon 10 Holston Medical Group Urgent Care-Kingsport 02 First Assist Urgent Care - Colonial Heights 11 MedExpress Urgent Care-Bluefield 03 First Assist Urgent Care - Elizabethton 12 MedExpress Urgent Care-Bristol 04 First Assist U
mproving State versight of Urgent Care Centers and Retail Health Clinics. 6. Licensing of Urgent Care Centers and Retail Health Clinics. URGENT CARE CENTERS. The vast majority of states do not issue facility licenses for urgent care centers. In the 40 states that have chosen not to issue such facility-specific licenses, most urgent care
“Cost accounting is a quantitative method that accumulates, classifies, summarizes and interprets information for three major purposes: (in) Operational planning and control ;( ii) Special decision; and (iii) Product decision.” -Charles T. Horngren. 2 “Cost accounting is the process of accounting for costs from the point at which the expenditure is incurred of committed to the .