Calcium And Vitamin D In Bone Fracture Healing And Post-traumatic Bone .
EuropeanV Fischer Cellset al. and Materials Vol. 35 2018 (pages 365-385)DOI: 10.22203/eCM.v035a25Calcium and vitaminISSN 1473-2262D in fracturehealingCALCIUM AND VITAMIN D IN BONE FRACTURE HEALING ANDPOST-TRAUMATIC BONE TURNOVERV. Fischer1, M. Haffner-Luntzer1, M. Amling2 and A. Ignatius1,*1Institute of Orthopaedic Research and Biomechanics, Ulm University, Trauma Research Centre Ulm,Ulm, Germany2Department of Osteology and Biomechanics, University Medical Centre Hamburg-Eppendorf,Hamburg, GermanyAbstractCalcium and vitamin D are essential for maintaining bone health. Therefore, deficiencies in calcium andvitamin D are major risk factors for osteoporosis development. Because sufficient amounts of calcium arealso required for fracture-callus mineralisation, compromised bone repair that is frequently observed inosteoporotic patients might be attributed to calcium and vitamin D deficiencies. Consequently, calcium andvitamin D supplementation represents a potential strategy for treating compromised fracture healing inosteoporotic patients. Growing clinical evidence suggests that a fracture event may induce post-traumaticbone loss in the non-fractured skeleton, particularly in osteoporotic patients, which might further exacerbateosteoporosis and increase the risk of secondary fractures. Because the skeleton represents the main sourceof calcium, which is increasingly required during fracture-callus mineralisation, post-traumatic calciummobilisation might occur under conditions of insufficient calcium and vitamin D status. However, to date,investigations of the roles of calcium and vitamin D in bone repair and post-traumatic bone turnover arevery limited.The current review summarises the state of the literature, focusing on the role of calcium and vitamin Din fracture healing and post-traumatic bone turnover, and critically discusses the therapeutic potential ofcalcium and vitamin D supplementation in this context.Keywords: Calcium, vitamin D, fracture healing, post-traumatic bone loss, osteoporosis.*Address for correspondence: Prof. Anita Ignatius, DVM, Institute of Orthopaedic Research and Biomechanics, University of Ulm, Helmholtzstraße 14, 89081 Ulm, Germany.Telephone number: 49 73150055301 Fax number: 49 73150055302 Email: anita.ignatius@uni-ulm.deCopyright policy: This article is distributed in accordance with Creative Commons Attribution 4.0/).List of min-D3alkaline phosphataseAmerican Society of Bone andMineral Researchbone gamma-carboxyglutamateproteinbone mineral densitycholecystokinin gastrin B receptorcytochrome P450 family 24 subfamilyA member oblast growth factor 23interleukinInternational dparathyroid hormonereceptor activator of NF-κBRANK ligandsecreted phosphoprotein 1tumour necrosis factor superfamilymember 11vitamin D receptorwww.ecmjournal.org
V Fischer et al.Calcium and vitamin D in fracture healingIntroductionOsteoporosis is globally the most common age-relatedskeletal disease, characterised by a progressivedecline in bone mass and disruption of the bonemicro-architecture, resulting in an increased risk offragility fractures. Numerous risk factors influenceosteoporosis development, including oestrogendecline during menopause, immobilisation, oldage and nutrition (Rachner et al., 2011). In respectto nutrition, major osteoporosis risk factors are aninsufficient calcium supply and a reduced vitamin Dstatus. Calcium is the main mineral present in bones,where it provides skeletal strength and serves as areservoir for maintaining blood calcium levels in aphysiological range. Vitamin D is the key controller ofcalcium homeostasis by regulating intestinal calciumabsorption, renal calcium reabsorption and boneremodelling (Amling et al., 1999; Li et al., 1997). Bothcalcium and vitamin D deficiencies promote boneloss through increased bone resorption in order tomaintain the blood calcium concentration (Lips andvan Schoor, 2011; Peacock, 2010). Vitamin D deficiencyis recognised as a global health problem and isexpected to increase further because of demographicchanges reflecting an aging population (Hosseinnezhad and Holick, 2013). Because of the importantroles of calcium and vitamin D in bone health, basicosteoporosis therapy includes their supplementationfor individuals at high risk of osteoporosis, includingaged postmenopausal females, when dietary calciumand vitamin D intake are insufficient (Cosman et al.,2014). However, osteoporosis treatment is rarelyapplied in clinics because osteoporosis is a silentdisease, which is primarily diagnosed after patientshave experienced the first fragility fracture. Even aftersuch a fracture, under-treatment is common, becauseonly 10-20 % of patients receive adequate treatment(Bellantonio et al., 2001; Follin et al., 2003).Both calcium and vitamin D play key roles in bonemineralisation, which is also part of the fracturehealing process (Claes et al., 2012; Einhorn andGerstenfeld, 2015). Therefore, it is likely that calciumand vitamin D deficiencies contribute to fracturehealing complications, which are frequently observedin osteoporotic, postmenopausal patients (Nikolaouet al., 2009). However, only limited experimentaland even fewer clinical studies investigate the roleof calcium and vitamin D in fracture healing, asreviewed here. In addition, only a few investigatepost-traumatic changes in the non-fractured skeleton,particularly under calcium- and vitamin-D-deficientconditions. There is growing clinical evidence ofsystemic bone loss following a fracture, as indicatedby a reduction in the total bone mass of 2-15 %,compared to values immediately post-fracture orage-matched controls without fracture (Fox et al.,2000; Karlsson et al., 2000). Systemic bone loss mayexplain the clinical observation of an increased riskof secondary fractures (Ahmed et al., 2013; Lyles etal., 2008). Experimental data suggest that calciumand vitamin D deficiencies might exacerbate posttraumatic bone loss (Fischer et al., 2017; HaffnerLuntzer et al., 2016). Hereby, calcium, which isrequired for fracture-callus mineralisation, isincreasingly mobilised from the remote skeleton toguarantee sufficient bone repair since the dietarycalcium supply does not meet calcium requirementsfor callus mineralisation. These findings imply aclinical therapeutic requirement for calcium andvitamin D supplementation after fracture.The scope of the current review was to summariseand analyse the known experimental and clinicaldata on the role of calcium and vitamin D in regularand osteoporotic fracture healing, as well as in posttraumatic bone turnover. Moreover, the therapeuticpotential of calcium and vitamin D supplementationin the clinic, in particular in the prevention of fracturehealing complications and post-traumatic bone loss,is discussed based on the reviewed literature.Bone remodellingBone is dynamically remodelled throughout theentire lifespan to replace damaged bone and adaptto mechanical load by the balanced and coupledactions of bone-forming osteoblasts and boneresorbing osteoclasts. Balanced bone remodellingis essential for the maintenance of bone mass andskeletal integrity. The complex process of boneremodelling is regulated by a variety of endogenousand exogenous factors. Primarily, it is regulatedthrough the RANK/RANKL/OPG system actingdirectly on osteoblast/stroma cells and osteoclastprecursors. RANKL, expressed by osteoblasts/stromacells, binds to the RANK receptor on osteoclastprecursors inducing osteoclastogenesis (Suda et al.,1999). Osteoblast-produced OPG functions as a decoyreceptor, blocking the effects of RANKL (Hsu et al.,1999). Many local and systemic factors regulatingbone remodelling – including transforming growthfactor-β, bone morphogenic proteins, cytokines-likeIL-1β, IL-6 and tumour necrosis factor-α, hormonessuch as PTH, 1,25-VitD3 and oestrogen – mainlysignal by influencing the RANK/RANKL/OPGsystem on osteoblasts/stroma cells, thus keeping thesystem in balance (Crockett et al., 2011). In additionto these endogenous determinants, exogenousfactors, including mechanical loading and nutrition,are involved. Osteocytes sense and respond tomechanical stimuli by the induction of osteo-anabolicsignals, including nitric oxide and prostaglandins(Robling et al., 2006). Calcium and vitamin D are themain nutrients exerting important functions in boneremodelling (Gennari, 2001).Imbalances in bone remodelling can drive bonemetabolism towards increased bone formation,favouring abnormal bone gain, as seen inosteosclerosis, or towards increased bone resorption,resulting in bone loss, as observed in osteoporosis.366www.ecmjournal.org
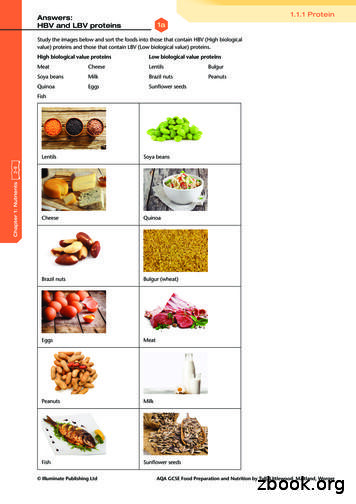
V Fischer et al.Calcium and vitamin D involvement in boneremodelling and homeostasisDietary composition, in particular, the amounts ofcalcium and vitamin D, play an important role in boneremodelling and skeletal integrity (Gennari, 2001).Approximately 99 % of the body’s calcium is present inbones and teeth, stored as hydroxyapatite responsiblefor tissue mineralisation. In bone, it provides skeletalstrength and serves as a calcium reservoir to maintainconstant blood calcium levels. Therefore, calciumis required for skeletal growth, development andmaintenance. Calcium requirements differ duringthe lifetime, depending on the varying needsdue to growth in childhood and youth, or duringpregnancy and lactation (Nordin, 1997). Calcium asan essential element is only available through thediet. However, dietary calcium recommendationsvary widely among countries. For example, therecommended daily calcium intake for the elderlyabove 65 years in Germany is 1000 mg, in the USA1200 mg and in the UK 700 mg (German NutritionSociety, 2013; Institute of Medicine, 2011; Prentice,2013). Differences might arise from variations in dataacquisition and interpretation and cultural aspects,including geographic locations, lifestyle and genetics.Under physiological conditions, approximately 3040 % of the ingested calcium is absorbed by the gut.Several factors influence calcium absorption rate,including total calcium amount, nature of the calciumcomplex, acidic conditions in the stomach and smallintestine, age and vitamin D status (Fleet, 2017;Krause et al., 2015; Schinke et al., 2009). In the case ofhigh dietary calcium intake, calcium is absorbed bypassive diffusion. By contrast, normal to low dietarycalcium intake requires active calcium absorption,which is regulated by the active 1,25-VitD3 actingon the VDR in the intestine (Christakos et al., 2014).Therefore, vitamin D plays a key role in calcium andbone homeostasis.The fat-soluble vitamin D (cholecalciferol) isingested as part of the diet or is synthesised in theskin upon ultraviolet-B irradiation (Holick et al.,1980). To acquire physiologic activity, vitamin Dis firstly hydroxylated in the liver on carbon 25 by25-hydoxylase, producing 25-VitD3 (calcifediol).In the kidney, 25-VitD3 is secondly hydroxylatedon carbon 1 by a 1-α-hydroxylase, producing thebiologically active 1,25-VitD 3 (calcitriol). In thetarget tissues, 1,25-VitD3 binds to its nuclear VDR,a family member of the steroid/thyroid hormonereceptors, and functions as a transcription factor. As aheterodimer mainly bound to the retinoid-X-receptor,the VDR binds to vitamin D response elements on theDNA, thus modulating the transcription of numerousvitamin D target genes, including the bone-relatedgenes osteocalcin (BGLAP), osteopontin (SPP1) andRANKL (TNFSF11). The VDR is expressed by mostmammalian organs and their respective cell types,including the immune system, gastro-intestinal tract,reproductive organs, kidney, parathyroid gland, skin,heart, brain, muscles and bones, thus suggesting aCalcium and vitamin D in fracture healingbroad variety of biological functions (Christakos et al.,2016). However, the key function of 1,25-VitD3/VDRis the regulation of mineral and bone homeostasis (Liet al., 1998).1,25-VitD 3, PTH and FGF23 (a key regulatorof phosphate and vitamin D metabolism) are themain factors maintaining constant blood calciumand phosphate levels by regulating: i) calciumand phosphate absorption in the intestine andreabsorption in the kidney, ii) bone resorption. Themain inducer of this complex regulatory network is achange in serum calcium levels, since constant calciumconcentrations are essential for many biologicalfunctions. The inactivation of the calcium-sensingreceptor in the parathyroid glands, resulting from afall in serum calcium levels, stimulates PTH release(Fig. 1). Circulating PTH binds to its receptor in thekidney, where it enhances calcium reabsorption,phosphate excretion and 1,25-VitD3 production. Bothcirculating PTH and 1,25-VitD3 bind their respectivereceptors on osteoblasts, thus increasing RANKLexpression, which stimulates osteoclastic boneresorption and the release of calcium and phosphateinto the circulation. Consequently, calcium levelsare restored and negative feedback mechanisms areinduced, including the release of calcitonin from thethyroid gland, which reduces calcium reabsorptionin the kidney and absorption in the gut and inhibitsosteoclastic bone resorption, thus maintainingcalcium levels in a physiological range (Fig. 1).The parallel increase of phosphate levels duringthe calcium-mediated PTH and 1,25-VitD3 actionsrequires the phosphate-lowering actions of FGF23,independent of PTH (Fig. 1). The 32 kDa FGF23,which belongs to the FGF19 subfamily, is increasinglyreleased by osteocytes in bones upon high circulatingphosphate and 1,25-VitD3 concentrations (Liu etal., 2006). In the kidney, FGF23, associated withits cofactor Klotho, binds the FGF1 receptor, thusdecreasing renal phosphate reabsorption andincreasing its excretion (Urakawa et al., 2006). Inaddition, FGF23 further reduces phosphate levels byinhibiting 1,25-VitD3 production in the kidney andprobably PTH production in the parathyroid gland(Ben-Dov et al., 2007; Shimada et al., 2004). Severalinherited syndromes characterised by abnormalitiesin FGF23 levels are associated with osteomalacia, thusstrengthening the role of FGF23 in bone homeostasis(Shimada et al., 2001; Weber et al., 2003; Yamazakiet al., 2002). In addition, as shown in experimentaland clinical studies, genetic Klotho deficiency isassociated with reduced bone mass (Kuro-o et al.,1997; Yamada et al., 2005; Zarrabeitia et al., 2007).In contrast, studies investigating associations ofserum Klotho and bone mass report controversialresults (Baldan et al., 2015; Chalhoub et al., 2016).In osteoporotic elderly, no associations betweenserum Klotho and bone loss and fracture risk aredetected (Chalhoub et al., 2016). Since the membranebound Klotho protein, which is required for FGF23function in bone, is not measurable in the blood, the367www.ecmjournal.org
V Fischer et al.relevance of the secreted Klotho protein as a markerfor osteoporosis is uncertain and needs to be furtherexamined.In conclusion, the process of bone remodelling isessential for adapting to the changes in calcium levels.However, a low dietary calcium supply or longlasting vitamin D deficiency, which considerablyreduces intestinal calcium absorption and increasesPTH concentrations, stimulates bone turnover andexcessive bone resorption to restore systemic calciumlevels. These mechanisms favour bone loss andosteoporosis development.Calcium and vitamin D in fracture healingCalcium and vitamin D in osteoporosisOsteoporosis is globally the most common skeletaldisease, affecting alone in Europe, USA and Japanan estimated 75 million people, as stated by the IOFin 1997 (Consensus Development Conference, 1997).Due to demographic changes of an aging population,the number has increased in the last decades and willfurther increase in the future. In 2010, an estimatednumber of 53.6 and 27.5 million people suffered ofosteoporosis in the USA and Europe, respectively(Hernlund et al., 2013; Wright et al., 2014). TheIOF estimates that more than 200 million womenFig. 1. Regulation of calcium and bone homeostasis. Upon a fall in serum calcium, PTH is secreted fromthe parathyroid glands. Circulating PTH increases calcium mobilisation from bones and stimulates bothcalcium reabsorption and 1,25-VitD3 synthesis in the kidneys. 1,25-VitD3 increases calcium absorption in thegut. In response to PTH and 1,25-VitD3 actions, phosphate levels increase, stimulating FGF23 secretion frombone osteocytes. In the kidney, FGF23 promotes phosphate excretion and inhibits 1,25-VitD3 production.FGF23 might further inhibit PTH secretion. Restored/high serum calcium levels trigger negative feedbackloops, including calcitonin acting on its own receptor, thus reducing renal calcium reabsorption andinhibiting osteoclastic bone resorption (illustrated in bright grey). Ca calcium; PO phosphate;PTH parathyroid hormone; 1,25-(OH)2D3 1,25-dihydroxy-vitamin D3; FGF23 fibroblast growth factor23; VDR vitamin D receptor; PTHR parathyroid hormone receptor; FGFR1 fibroblast growth factorreceptor 1; CaR calcium sensing receptor; CAR calcitonin receptor.368www.ecmjournal.org
V Fischer et al.worldwide suffer from osteoporosis (Kanis, 2007). Thedisease is characterised by a progressive decline inbone mass and disruption of bone microarchitecturebecause of the unbalanced activities of osteoclastsand osteoblasts, where bone resorption exceedsbone formation. According to the World HealthOrganization, osteoporosis is defined by a reductionin BMD of 2.5 or more standard deviations below themean BMD of young adults (Cosman et al., 2014).Osteoporosis is associated with an increased risk offragility fractures, mainly occurring at the spine, hipand wrist. The lifetime risk of an osteoporotic fractureis 40 % in females versus 27 % in males (Cooley andJones, 2001; Kanis et al., 2000). The most commonform is postmenopausal osteoporosis, whichresults from a decline in oestrogen levels because ofmenopause, and globally affects an estimated 200million females (Kanis, 2007). Overall, a panel of riskfactors, including genetics, gender, age, comorbiditiesand their therapies, life style and nutrition influenceosteoporosis development.Calcium and vitamin D deficiencies representthe main risk factors influencing osteoporosisdevelopment. Changes in serum calcium entailadaptations in bone remodelling, as in the case ofincreased bone resorption induced by low serumcalcium. With a mean daily calcium intake ofapproximately 700-800 mg in adults in Germany(European Food Safety Authority, 2006), the calciumsupply remains slightly below the recommendationof 1000 mg/d (German Nutrition Society, 2013).However, both intestinal calcium absorption anddietary calcium intake decrease with increasingage (Ireland and Fordtran, 1973; Wakimoto andBlock, 2001). Such low-to-normal dietary calciumintake requires active intestinal transport by 1,25VitD 3 (McCormick, 2002). However, vitamin Ddeficiency is common (Holick and Chen, 2008). Onemain reason is the low dietary vitamin D intake,because only a few foods have a high vitamin Dcontent, including oily fish, eggs, milk and somedairy products. Because eating habits change withage, the lowest vitamin D supply from the diet is inthose above 65 years (Wakimoto and Block, 2001).However, endogenous vitamin D synthesis in theskin upon sunlight exposure supplies 80-90 % of therequired vitamin D. Nevertheless, numerous factors,including geographic location, latitude, season,time spent outdoors and clothing, influence dermalvitamin D synthesis. For example, solar irradiationduring winter is insufficient to produce vitamin Din people resident in northern countries (Webb et al.,1988). To guarantee optimal bone metabolism andbone health, systemic 25-VitD3 levels of 75 nmol/Lor more ( 30 ng/mL) are required, because lower25-VitD3 concentrations are associated with bonemineralisation defects (Priemel et al., 2010; vonDomarus et al., 2011). Globally, it is estimated thatvitamin D deficiency affects approximately 3 billionpeople (Hossein-nezhad and Holick, 2013). In Europe,roughly 80 % of the population displays insufficientCalcium and vitamin D in fracture healing25-VitD3 serum levels below 75 nmol/L ( 30 ng/mL) (Gonzalez-Gross et al., 2012; Holick et al., 2011).Particularly in Germany, widespread vitamin Ddeficiency is common and occurs through all ages(Hintzpeter et al., 2008a; Hintzpeter et al., 2008b).However, the effects are even more pronounced inthe elderly (Schilling, 2012), due to reduced dermalvitamin D-synthesis capacity with age (MacLaughlinand Holick, 1985) and reduced sunlight exposurebecause of immobility and less time spent outdoor(Bruyere et al., 2014; Holick et al., 1980). Indeed, inGermany, the mean 25-VitD3 levels are 46.2 nmol/Lin adults and 39.1 nmol/L over 65 years (GermanNutrition Society, 2012). Particularly in risk groups,including elderly and postmenopausal females,severe vitamin D deficiency might interfere withbone health, because of reduced intestinal calciumabsorption capacity. Indeed, several epidemiologicalstudies demonstrate associations between low 25VitD3 levels and reduced bone mass on one sideand increased risk of falls and fragility fractureson the other (Bischoff-Ferrari et al., 2014; Kuchuket al., 2009). Therefore, preventive and therapeuticactions, including nutrient supplementation andfood fortification, ensuring minimum circulating 25VitD3 levels (75 nmol/L or 30 ng/mL) are required topreserve and restore bone health (Brown et al., 2013a;Brown et al., 2013b).For osteoporosis and fracture prevention,practical guidelines of the National OsteoporosisFoundation recommend calcium and vitamin Dsupplementation for high-risk groups, includingpostmenopausal females ( 50 years old), whendietary calcium and vitamin D intake is insufficient(Cosman et al., 2014). In addition, calcium and vitaminD supplementation is, as basal therapy, alwayspart of other osteoporosis treatments, includingbisphosphonates, intermittent PTH applicationor novel drugs targeting sclerostin, RANKL orcathepsin K (Rachner et al., 2011; Tabatabaei-Malazyet al., 2017). However, the efficiency and safety ofcalcium, vitamin D or combined calcium and vitaminD supplementations are critically discussed. On theone hand, several clinical trials observe a reductionin hip and non-vertebral fractures because of calciumand vitamin D supplementation (Bischoff-Ferrariet al., 2008; Bischoff-Ferrari et al., 2005; Chapuy etal., 1992; Warensjo et al., 2011). In addition, a metaanalysis by Tang et al. (2007) report that calcium andvitamin D supplementation reduce osteoporoticfracture occurrence, thus corroborating the beneficialeffects of calcium and vitamin D supplementationon fracture risk reduction. On the other hand, somemeta-analyses do not observe associations betweencalcium and vitamin D supplementation andfracture-risk reduction (Bolland et al., 2015; Peacocket al., 2000; Zhao et al., 2017). However, most ofthese studies are conducted in healthy communitydwelling adults, who clearly do not profit fromcalcium or vitamin D supplements. By contrast,risk groups of elderly, particularly postmenopausal369www.ecmjournal.org
V Fischer et al.Calcium and vitamin D in fracture healingfemales, displaying reduced calcium intake andinsufficient vitamin D status benefit from calciumand vitamin D supplements (Harvey et al., 2017;Tang et al., 2007). Confirming this, a very recentlypublished statement of the ASBMR warns of reportshighlighting no beneficial effects of calcium andvitamin D supplementation in community-dwellingadults, because such meta-analyses frequently focusonly on healthy adults. The ASBMR clearly state thatthese findings do not apply to osteoporotic patientsor patients taking bone protective drugs, includingbisphosphonates and PTH, because calcium andvitamin D supplementation, as a part of a basaltherapy, influences drug efficiency and treatmentsuccess (Web ref. 1). However, a general populationbased treatment with calcium and vitamin D is notrecommended, because beneficial health effects are, todate, not demonstrated (Harvey et al., 2017). Sufficientcalcium and vitamin D should be obtained throughthe diet or skin synthesis. However, when this is notachievable, supplements are recommended for riskgroups (Cosman et al., 2014). To exclude criticallydiscussed and still scientifically unproven side effectsof increased calcium levels due to supplementation– which might favour the development of kidneystones, cardiovascular diseases or gastrointestinalsymptoms – patients with a history of these diseasesshould be monitored for serum parameters of calciummetabolism. However, the tolerable upper calciumlimit posing no risk of adverse health effects for thegeneral population is 2500 mg/d, which is not reachedby the dietary supply or calcium supplements,commonly containing 500-1200 mg of calcium(European Food Safety Authority, 2006).Osteoporosis is frequently under-treated andunder-diagnosed until patients have experiencedthe first osteoporotic fracture. Even after such afracture, only 10-20 % of patients receive adequateosteoporosis treatment, including calcium andvitamin D supplementations (Bellantonio et al.,2001; Follin et al., 2003; Weaver et al., 2017). Themain reasons are a lack of diagnosis and therapyinitiation, possibly because of side effects, uncertainefficiencies, high costs and patient’s health status,compliance and motivation. In addition, the primarygoal of trauma surgeons is fracture stabilisation andreduction, which might be one reason for the lackof diagnosis and treatment initiation (Weaver et al.,2017). Interdisciplinary approaches strengtheningthe collaboration of trauma surgeons, osteologistsand nutritionists might reduce the existing therapydeficit, which may further reduce the risk of fragilityfractures.Fracture healingFractures caused by osteoporosis occur in one third offemales and one fifth of males over 50 years. Globally,approximately 9 million people with osteoporosissuffer a fracture annually (Johnell and Kanis, 2006).Thus, because of ongoing demographic changeswith an aging population, the incidence of fractureswill further increase. Bone fractures normally healwithout complications and any scar formation.However, under specific conditions, including oldage, impaired health status, comorbidities andsevere injuries/traumas, the repair process may fail(Claes et al., 2012). Therefore, a total of 5-10 % of allfractures display disturbed bone healing (Zura etal., 2016). Bone fracture healing is a highly dynamic,complex and tightly regulated process that involvesthe interplay of many cells and molecular mediators,including growth factors and cytokines, and isfurther influenced by the biomechanical environmentof the fracture-healing zone. The repair processproceeds in three overlapping phases: inflammation,repair and remodelling. The inflammatory phase ischaracterised by tissue and cell damage, rupture ofblood vessels and recruitment of immune cells tothe fracture haematoma. During the repair phase,intramembranous and endochondral ossificationdrive fracture-callus formation and growth towardsthe fracture gap until bony bridging. Duringremodelling, the newly formed woven bone isreplaced by lamellar bone, thus restoring the originalbone structure and stability (Claes et al., 2012). Thechanges that occur with the onset of osteoporosismight interfere with this complex and tightlyregulated repair process. Indeed, compromised bonerepair is frequently observed in osteoporotic patients(Cornell et al., 2003; Nikolaou et al., 2009; Zura et al.,2016). However, pathomechanisms are still poorlyunderstood and further research is needed.Osteoporotic fracture healingOsteoporotic fractures are more frequently associatedwith complications, including infections andimplant failure, resulting in expensive aftercare withprolonged hospitalisation periods and increasedmorbidity and mortality rates. Implant failureoccurs in approximately 10-25 % of osteoporoticfracture cases (von Ruden and Augat, 2016). Thetotal complication rate, not only including bonerelated complications but also infections, pneumoniaand anaemia, is approximately 60 % after hip and50 % after vertebral fractures and increases withage (Lehmann et al., 2016). Nikolaou et al. (2009)demonstrate that fracture-healing time is significantlyprolonged in older osteoporotic patients, thusindicating a delay of the repair process. Furthermore,half of the osteoporotic patients do not fully recoverafter hip injury (Cornell et al., 2003). Epidemiologicalanalysis show that the risk of fracture non-union issignificantly increased in osteoporotic patients (Zuraet al., 2016). Therefore, osteoporosis affects the boneregenerative capacity, resulting in fracture-healingcomplications. However, it is still strongly debatedwhether osteoporotic bones heal worse because ofpoor fixation stability in fragile bone or whether the370www.ecmjournal.org
V Fischer et al.Calcium and vitamin D in fracture healingTable 1. Summary of experimental and clinical studies on the effects of calcium and vitamin D on fracturehealing. s.c.: subcutaneous; i.m.: intramuscular.Study and fracture typeTreatmentFracture-healing outcomeAuthorsExperimental studiesMale rats (age: 2 weeks)hind leg fractureCalcium- and/orphosphorus-deficientdietImpaired healing: callusmineralisationDoepfner, 1970Male ratsfemur drill hole defectInjection (s.c.) of 1,25VitD3Improved healing: biomechanical propertiesLindgren et al., 1981Male rats (age: mature)femur fracture,intramedullary fixationCalcium-/vitaminD-deficient dietCalcium-/vitaminD-supplemented dietMale guinea pigstibia fractureSingle high-doseinjection (i.m.) ofvitamin DImproved healing: blood supply,callus formation andmineralisationOmeroglu et al.,1997bMale rabbits (age: 3months)femur fracture,intramedullary fixatio
Therefore, vitamin D plays a key role in calcium and bone homeostasis. The fat-soluble vitamin D (cholecalciferol) is ingested as part of the diet or is synthesised in the skin upon ultraviolet-B irradiation (Holick et al., 1980). To acquire physiologic activity, vitamin D is firstly hydroxylated in the liver on carbon 25 by
Vitamin A Keeps the skin healthy Helps us see in dim light Helps children to grow Keeps mucous membranes moist and healthy This vitamin is an antioxidant Vitamin D Helps calcium to be absorbed in the body Helps calcium to strengthen the bones and teeth Vitamin E This vitamin is an antioxidant Vitamin K Helps the blood.
Konsumsi asam folat, vitamin B12 dan vitamin C pada ibu hamil tergolong masih rendah, sehingga konsumsi sumber vitamin perlu ditingkatkan untuk mencegah masalah selama kehamilan, seperti anemia, prematur, dan kematian ibu dan anak. Kata kunci: asam folat, ibu hamil, vitamin B12, vitamin C *Korespondensi: Telp: 628129192259, Surel: hardinsyah2010@gmail.com J. Gizi Pangan, Volume 12, Nomor 1 .
Milk Thistle Red Clover Rhodiola St. John’s Wort Soy Bean Tomato Tribulus Terrestris Willow Vitamin B1 Vitamin B2 Vitamin B6 Vitamin B12 Vitamin C Vitamin D3 Vitamin E MISCELLANEOUS Alpha Lipoic Acid Beta Carotene Caffeine Choline Bitartrate Chond. Sulphate Bovine Chond. Sulphate Porcine Ch
Normal vitamin D 36% 9% 55% Vitamin D deficiency* Severe vitamin D deficiency** Normal vitamin D Camargo CA, Jr., Ingham T, Wickens K, et al. Vitamin D status of newborns in New Zealand. Br J Nutr 2010;104:1051 -7. Grant CC, Wall CR, Crengle S, Scragg R. Vitamin D deficiency in early childhood Public Health Nutr. 2009;12(10):1893-1901
vitamin D deficiency will have a depleted calcium status and/or a poor calcium intake and may therefore benefit from advice about dietary calcium intake. In some cases calcium supplementation may be worthwhile over the period of vitamin D treatment (see Appendix 1). These recommendations
VITAMIN A This vitamin helps your body maintain healthy eyes and skin. VITAMIN C This vitamin helps the body heal cuts and wounds and maintain healthy gums. VITAMIN E This vitamin helps maintain healthy cells throughout your body. WATER Water makes up more than half of your body weight. Your
25-OH Vitamin D levels* To determine vitamin D status * Only measure if patient is symptomatic and has risk factors for Vitamin D deficiency. Measurement, status and management (see Appendix 1 for flowchart) Vitamin D level Vitamin D status Health effect Management 30 nmol/L Defi
important.1 But the form of the vitamin D in it is. Look for supplements that contain: Vitamin D3, which is superior at optimizing and maintaining vitamin D levels long-term2 3 Or, if you prefer a plant-based option: Vitamin D2, which is derived from yeast or mushrooms For best absorption, take vitamin D with a meal, especially one that .