Element Name Symbol Atomic Number (not The Decimal
Summer 2017ElementsHonors ChemistryPlease fill out the chart using the periodic table on the bottom of this paper. You will need to have all the information onthe table memorized by the first day of school. Spelling counts Element NameSymbolAtomic Number(not the decimal number)Element diumSulfurTinUraniumZinc1SymbolAtomic Number(not the decimalnumber)
Units of MeasureHow were sailors able to measure the depths of seas?Back in the days before all the electronic gadgets for measuringdepth and locating undersea objects existed, the "fathom" wasthe unit of measurement for depth. A rope was knotted every sixfeet and the end was dropped over the side of the ship. You couldtell how deep the water was by how many knots went under thewater before the rope hit bottom. Today, we just turn on aninstrument and read the depth to a high level of accuracy.Length and VolumeLength is the measurement of the extent of something along its greatest dimension. The SI basic unit of length, orlinear measure, is the meter (m). All measurements of length may be made in meters, though the prefixes listed invarious tables will often be more convenient. The width of a room may be expressed as about 5 meters (m),whereas a large distance, such as the distance between New York City and Chicago, is better expressed as 1150kilometers (km). Very small distances can be expressed in units such as the millimeter or the micrometer. The widthof a typical human hair is about 20 micrometers (μm).Volume is the amount of space occupied by a sample of matter. The volume of a regular object can be calculatedby multiplying its length by its width by its height. Since each of those is a linear measurement, we say that units ofvolume are derived from units of length. The SI unit of volume is the cubic meter (m3), which is the volumeoccupied by a cube that measures 1 m on each side. This very large volume is not very convenient for typical use ina chemistry laboratory. A liter (L) is the volume of a cube that measures 10 cm (1 dm) on each side. A liter is thusequal to both 1000 cm3 (10 cm 10 cm 10 cm) and to 1 dm3. A smaller unit of volume that is commonly used isthe milliliter (mL – note the capital L which is a standard practice). A milliliter is the volume of a cube that measures1 cm on each side. Therefore, a milliliter is equal to a cubic centimeter (cm3). There are 1000 mL in 1 L, which is thesame as saying that there are 1000 cm3 in 1 dm3.To determine the volume of a liquid, usually in a graduated cylinder, you look at the bottom curve which is calledthe meniscus, the curve seen at the top of a liquid in response to its container. When you read a scale on the sideof a container with a meniscus, such as a graduated cylinder or volumetric flask, it's important that themeasurement accounts for the meniscus. Measure so that the line you are reading is evenwith the center of the meniscus. For water and most liquids, this is the bottom of themeniscus.1
Units of MeasureMass and WeightHow is he floating?One of the many interesting things about travel in outer space isthe idea of weightlessness. If something is not fastened down, itwill float in mid-air. Early astronauts learned that weightlessnesshad bad effects on bone structure. If there was no pressure onthe legs, those bones would begin to lose mass. Weightprovided by gravity is needed to maintain healthy bones.Specially designed equipment is now a part of every spacemission, so the astronauts can maintain good body fitness.Mass is a measure of the amount of matter that an objectcontains. The mass of an object is made in comparison to the standard mass of 1 kilogram. The kilogram wasoriginally defined as the mass of 1 L of liquid water at 4 C (volume of a liquid changes slightly with temperature). Inthe laboratory, mass is measured with an electric balance which must be calibrated with a standard mass so that itsmeasurements are accurate.Other common units of mass are the gram and the milligram. A gram is 1/1000th of a kilogram, meaning that thereare 1000 g in 1 kg. A milligram is 1/1000th of a gram, so there are 1000 mg in 1 g.Mass is often confused with the term weight. Weight is a measure of force thatis equal to the gravitational pull on an object. The weight of an object isdependent on its location. On the moon, the force due to gravity is about onesixth that of the gravitational force on Earth. Therefore, a given object willweigh six times more on Earth than it does on the moon. Since mass isdependent only on the amount of matter present in an object, mass does notchange with location. Weight measurements are often made with a spring scaleby reading the distance that a certain object pulls down and stretches a spring.Temperature and Temperature ScalesTouch the top of the stove after it has been on, and itfeels hot. Hold an ice cube in your hand and it feels cold.Why? The particles of matter in a hot object are movingmuch faster than the particles of matter in a cold object. An object’s kinetic energy is the energy due to motion.The particles of matter that make up the hot stove have a greater amount of kinetic energy than those in the icecube.Temperature is a measure of the average kinetic energy of the particles in matter. In everyday usage, temperatureindicates a measure of how hot or cold an object is. Temperature is an important parameter in chemistry. When asubstance changes from solid to liquid, it is because there was an increase in the temperature of the material.Chemical reactions usually proceed faster if the temperature is increased. Many unstable materials (such asenzymes) will be viable longer at lower temperatures.2
Units of MeasureTemperature ScalesThe first thermometers were glass and contained alcohol, which expanded and contracted asthe temperature changed. The German scientist, Daniel Gabriel Fahrenheit, used mercury inthe tube. The Fahrenheit scale was first developed in 1724 and tinkered with for some timeafter that. The main problem with this scale is the arbitrary definitions of temperature. Thefreezing point of water was defined as 32 F and the boiling point as 212 F. The Fahrenheitscale is typically not used for scientific purposes.Daniel Gabriel FahrenheitThe Celsius scale of the metric system is named after Swedish astronomer AndersCelsius (1701-1744). The Celsius scale sets the freezing point and boiling point of waterat 0 C and 100 C respectively. The distance between those two points is divided into100 equal intervals, each of which is one degree. Another term sometimes used for theCelsius scale is “centigrade” because there are 100 degrees between the freezing andboiling points of water on this scale. However, the preferred term is “Celsius.”Anders CelsiusThe Kelvin temperature scale is named after Scottish physicist and mathematician Lord Kelvin(1824-1907). It is based on molecular motion, with the temperature of 0 K, also known asabsolute zero, being the point where all molecular motion ceases. The freezing point of wateron the Kelvin scale is 273.15 K, while the boiling point is 373.15 K. Notice that here is no“degree” used in the temperature designation. Unlike the Fahrenheit and Celsius scales wheretemperatures are referred to as “degrees F” or “degrees C,” we simply designatedtemperatures in the Kelvin scale as kelvins.Lord KelvinAs can be seen by the 100 kelvin difference between the two, (boiling points and freezingpoint), a change of one degree on the Celsius scale is equivalent to the change of one kelvin on theKelvin scale. Converting from one scale to another is easy, as you simply add 273 to gofrom Celsius to Kelvin or subtract 273 to go from Kelvin to Celsius.3
Metric SystemMany properties of matter are quantitative; that is, they are associated with numbers. When a number representsa measured quantity, the unit of that quantity must always be specified. To say that the length of a pencil is 17.5 ismeaningless; however, saying it is 17.5 cm specifies the length. The units used for scientific measurements arethose of the metric system.The metric system was developed in Franceduring the late 1700s and is the most commonform of measurement in the world. There are afew countries, The United States of America, thatdo not follow the metric system; we use theEnglish system. Over the years the use of themetric system has become more common; justlook at a can of soda, there are indications of themetric system.Metric PrefixesConversions between metric system units arestraightforward because the system is based onpowers of ten. For example, meters, centimeters,and millimeters are all metric units of length.There are 10 millimeters in 1 centimeter and 100centimeters in 1 meter. Metric prefixes are used todistinguish between units of different size. Theseprefixes all derive from either Latin or Greek terms.Can of Soda showing both English (oz) & Metric (mL) unitsThe tables above lists the most common metric prefixes and their relationship to the central unit that has no prefix.There are a couple of odd little practices with the use of metric abbreviations. Most abbreviations are lower-case.We use “m” for meter and not “M”. However, when it comes to volume, the base unit “liter” is abbreviated as “L”and not “l”. So we would write 3.5 milliliters as 3.5 mL.As a practical matter, whenever possible you should express the units in a small and manageable number. If youare measuring the weight of a material that weighs 6.5 kg, this is easier than saying it weighs 6500 g or 0.65 dag. Allthree are correct, but the kg units in this case make for a small and easily managed number. However, if a specificproblem needs grams instead of kilograms, go with the grams for consistency.4
Metric SystemConverting (Dimensional Analysis)How can a number of track laps be converted to a distance in meters?You are training for a 10-kilometer run by doing laps on a 400-meter track. You ask yourself“How many times do I need to run around this track in order to cover ten kilometers?” (Morethan you realize & one of the many reasons I don’t run). By using dimensional analysis, you caneasily determine the number of laps needed to cover the 10 k distanceConversion FactorsMany quantities can be expressed in several different ways. The English of system measurementof 4 cups is also equal to 2 pints, 1 quart, and 0.25 of a gallon.4 cups 2 pints or 1 quart or 0.25 gallonNotice that the numerical component of each quantity is different, while the actual amount of material that itrepresents is the same. That is because the units are different. We can establish the same set of equalities for themetric system:1 meter 10 decimeters or 100 centimeters or 1000 millimetersThe metric system’s use of powers of 10 for all conversions makes this quite simple.Whenever two quantities are equal, a ratio can be written that is numerically equal to 1. Using the metric examplesabove:1m 100cm 1m 1100cm1000mm1mThe 1 m/100 cm is called a conversion factor. A conversion factor is a ratio of equivalent measurements. Becauseboth 1 m and 100 cm represent the exact same length, the value of the conversion factor is 1. The conversionfactor is read as “1 meter per 100 centimeters”. Other conversion factors from the cup measurement example canbe:4 cups 2 pints2 pints 1 quart1 quart¼ gallon 1Since the numerator and denominator represent equal quantities in each case, all are valid conversion factors.Scientific Dimensional AnalysisConversion factors are used in solving problems in which a certain measurement must be expressed with differentunits. When a given measurement is multiplied by an appropriate conversion factor, the numerical value changes,but the actual size of the quantity measured remains the same. Dimensional analysis is a technique that uses theunits (dimensions) of the measurement in order to correctly solve problems. Dimensional analysis is best illustratedwith an example.5
Metric SystemSet-Up# unit looking for Given x Unknown1Conversion factor The unit you are looking for MUST match the unit for your unknown. The unit for your given MUST match the uniton the conversion factorSample Problem 1:How many seconds are in a day?Step 1: List the known quantities and plan theproblem.Known 1 day 24 hours 1 hour 60 minutes 1 minute 60 secondsUnknown 1 day ? secondsThe known quantities above represent the conversion factors that we will use. The first conversion factor will haveday in the denominator so that the “day” unit will cancel. The second conversion factor will then have hours in thedenominator, while the third conversion factor will have minutes in the denominator. As a result, the unit of thelast numerator will be seconds, and that will be the units for the answer.Step 2: Calculate# secs 1 day x 24 hours x 60 min x 60 sec 86,400 sec1 day1 hour1 minApplying the first conversion factor, the “day” unit cancels and 1 x 24 24. Applying the second conversion factor,the “hour” unit cancels and 24 x 60 1440. Applying the third conversion factor, the “min” unit cancels and 1440 x60 86,400. The unit that remains is “s” for seconds.Step 3: Think about your result.Seconds is a much smaller unit of time than a day, so it makes sense that there are a very large number of secondsin one day.6
Metric SystemMetric Unit ConversionsThe metric system’s many prefixes allow quantities to be expressed in many different units. Dimensional analysis isuseful to convert from one metric system unit to another.Sample Problem 2:A particular experiment requires 120 mL of a solution. The teacher knows that he will need to make enoughsolution for 40 experiments to be performed throughout the day.How many liters of solution should he prepare?Step 1: List the known quantities and plan the problem.Known 1 experiment requires 120 mL 1 L 1000 mLUnknown L of solution for 40 experimentSince each experiment requires 120 ml of solution and the teacher needs to prepareenough for 40 experiments, multiply 120 by 40 to get 4800 mL of solution needed.Now you must convert ml to L by using a conversion factor.Step 2: Calculate# L 4800 mL x1L 4.8 L1000 mLNote that conversion factor is arranged so that the mL unit is in the denominator and thus cancels out, leaving L asthe remaining unit in the answer.Step 3: Think about your result.A liter is much larger than a milliliter, so it makes sense that the number of liters required is less than the numberof milliliters.7
Metric SystemTwo-Step Metric Unit ConversionsSome metric conversion problems are most easily solved by breaking them down into more than one step. Whenboth the given unit and the desired unit have prefixes, one can first convert to the simple (un-prefixed) unit,followed by a conversion to the desired unit. An example will illustrate this method.Sample Problem 3: Two-Step Metric ConversionConvert 4.3 km to cm.Step 1: List the known quantities and plan the problem.Known 1 m 100 cm 1 km 1000 mUnknown 4.3 cm ? kmYou may need to consult a table for the multiplication factor represented by each metric prefix. First convert cm tom, followed by a conversion of m to km.Step 2: Calculate# of cm 4.3 km x 1000 m x 100 cm 430,000 cm1 km1mEach conversion factor is written so that unit of the denominator cancels with the unit of the numerator of theprevious factor.Step 3: Think about your result.A centimeter is a smaller unit of length than a kilometer, so the answer in centimeters is larger than the number ofkilometers given.8
Metric SystemThe Magic SentenceThere are many tools that can be used to make your life in chemistry easier; one is the magic sentence to learn themetric prefixes and their values. And it goes like this:King Hector Died Monday Drinking Chocolate MilkKing (kilo)Hector (hector)Died (deca/deka)Monday (meter/gram/liter)Drinking (deci)Chocolate (centi)Milk (milli)Scientific NotationHow far is the Sun from Earth?Astronomers are used to really big numbers. While the moon is only 406,697km from earth at its maximum distance, the sun is much further away (150million km). Proxima Centauri, the star nearest the earth, is 39, 900,000, 000, 000 km away and we have just started on long distances. On theother end of the scale, some biologists deal with very small numbers: a typicalfungus could be as small as 30 μmeters (0.000030 meters) in length and a virusmight only be 0.03 μmeters (0.00000003 meters) long.Scientific NotationScientific notation is a way to express numbers as the product of two numbers: a coefficient and the number 10raised to a power. It is a very useful tool for working with numbers that are either very large or very small. As anexample, the distance from Earth to the Sun is about 150,000,000,000 meters –a very large distance indeed. Inscientific notation, the distance is written as 1.5 x 1011 m. The coefficient is the 1.5 and must be a number greaterthan or equal to 1 and less than 10. The power of 10, or exponent, is 11 because you would have to multiply 1.5 by1011 to get the correct number. Scientific notation is sometimes referred to as exponential notation.When working with small numbers, less than zero, we use a negative exponent. So 0.1 meters is 1 x 10-1 meters.Note the use of the leading zero (the zero to the left of the decimal point). That digit is there to help you see thedecimal point more clearly. The figure 0.01 is less likely to be misunderstood than .01 where you may not see thedecimal. When working with large numbers, greater than zero, we use a positive exponent. So 10 meters is 1.0 x101.The exponent represents the number of places the decimal point moves, not the number of zeroes in the number.If you move the decimal place to the left you add to the exponent the same number of places you moved; if you aremoving the decimal to the right you subtract from the exponent the same number of places you moved. This isoften referred to as LARS, (left – add and right – subtract).9
Summer 2017MetricsHonors ChemistryWorking with SI (metric) UnitsFor each of the following commonly used measurements, indicate its ondsUse the symbols to complete the following sentences with the most appropriate unit.1. The mass of a bowling ball is 7.25 .2. The lung capacity of an average man is about 4.8 .3. The length of a housefly is about 1 .4. The average length of time it takes to blink is about 2 .5. One teaspoon of cough syrup has a volume of 5 .6. The length of a human’s small intestine is about 6.25 .7. The mass of a paperclip is about 1 .8. When resting, the average adult’s heart beats once every 1.2 .9. The mass of a flea is about 0.5 .10. The distance between San Antonio and Dallas is approximately 440 .Write the abbreviation for the following common metric GramLiterDeciCentiMilli2
Summer 2017MetricsHonors ChemistryDimensional AnalysisConvert the following1. 35 daL dL11. 25 cm mm2. 950 g kg12. 0.005 kg dag3. 275 mm cm13. 0.075 m cm4. 1,000 L kL14. 15 g mg5. 1,000 mL L15. 0.987 kL hL6. 0.17 cm hm16. 1.281 mm m7. 2.65 km dm17. 12.07 hg dag8. 1.0 km mm18. 1625.0 cm m9. 18 dag cg19. 3017.36 mg dg10. 4,500 mg g20. 71.18 L cL3
Summer 2017Scientific NotationHonors ChemistryWrite the number(s) given in each problem using scientific notation. Don’t forget the unit.1. The human eye blinks an average of 4,200,000 times a year.2. A computer processes a certain command in 15 nanoseconds. (A nanosecond is one billionth of a second.) Indecimal form, this number is 0. 000 000 0153. There are 60,000 miles (97,000 km) in blood vessels in the human body. miles km4. The highest temperature produced in a laboratory was 920,000,000 F (511,000,000 C) at the Tokomak FusionTest Reactor in Princeton, NJ, USA. oF oC5. The mass of a proton is 0.000 000 000 000 000 000 000 001 673 grams.6. The mass of the sun is approximately 1,989,000,000,000,000,000,000,000,000,000,000 grams.7. The cosmos contains approximately 50,000,000,000 galaxies.8.A plant cell is approximately 0.00001276 meters wide.Write the number(s) given scientific notation in standard form. Don’t forget the unit.9. The age of earth is approximately 4.5 X 109 years.10. The weight of one atomic mass unit (a.m.u.) is 1.66 x 10-27 kg.4
Uncertainty in MeasurementHow do police officers identify criminals?After a bank robbery has been committed, police will askwitnesses to describe the robbers. They will usually get someanswer such as “medium height.” Others may say “between 5foot 8 inches and 5 foot 10 inches.” In both cases, there is asignificant amount of uncertainty about the height of thecriminals.Measurement UncertaintyThere are two types of numbers in the scientific world, exactnumbers and inexact numbers. Exact numbers are numberswhose values are known exactly. For example, there are 12 in adozen and 1000 grams in 1 kg.Inexact numbers have values with some uncertainty. If you give 10 students each a dime and tell them touse a triple beam balance to obtain the mass of their dime, you will slightly varying masses. The reason forthe differences may be due to equipment error, the balances not being calibrated equally, or human error,reading the balance wrong. Uncertainties always exist in measured quantities. The amount of uncertaintydepends both upon the skill of the measurer and upon the quality of the measuring tool. While somebalances are capable of measuring masses only to the nearest 0.1 g, other highly sensitive balances arecapable of measuring to the nearest 0.001 g or even better. Many measuring tools such as rulers andgraduated cylinders have small lines which need to be carefully read in order to make a measurement.The figure to the left shows two rulers making the same measurement of an object (indicated by the arrow).With either ruler, it is clear that the length of the object is between 2 and 3 cm. The bottom ruler contains nomillimeter markings. With that ruler, the tenths digit can be estimated and the length may be reported as 2.5cm. However, another person may judge that the measurement is 2.4 cm or perhaps 2.6 cm. While the 2 isknown for certain, the value of the tenths digit is uncertain.The top ruler contains marks for tenths of a centimeter (millimeters). Nowthe same object may be measured as 2.55 cm. The measurer is capableof estimating the hundredths digit because he can be certain that thetenths digit is a 5. Again, another measurer may report the length to be2.54 cm or 2.56 cm. In this case, there are two certain digits (the 2 and the5), with the hundredths digit being uncertain. Clearly, the top ruler is asuperior ruler for measuring lengths as precisely as possible.Precision and AccuracyThe terms precision and accuracy are often used indiscussing the uncertainties of measured values. Precisionis the measure of how closely individual measurementsagree with one another. Accuracy refers to how closelyindividual measurements agree with the correct or “true”value. Refer to figure above for a visual representation ofprecision and accuracy.10
Uncertainty in MeasurementSignificant FiguresHow fast do you drive?As you enter the town of Jacinto City, Texas, the sign below tells youthat the speed limit is 30 miles per hour. But what if you happen to bedriving 31 miles an hour? Are you in trouble? Probably not, becausethere is a certain amount of leeway built into enforcing the regulation.Most speedometers do not measure the vehicle speed very accuratelyand could easily be off by a mile or so (on the other hand, radarmeasurements are much more accurate). So, a couple of miles/hourdifference won’t matter that much. Just don’t try to stretch the limitsany further unless you want a traffic ticket.The significant figures in a measurement consist of all the certain digits inthat measurement plus one uncertain or estimated digit. In the ruler illustrationbelow, the bottom ruler gives a length with 2 significant figures, while the topgives a length with 3 significant figures. In a correctly reported measurement,the final digit is significant but not certain. Insignificant digits are not reported.With either ruler, it would not be possible to report the length as 2.553 cm asthere is no possible way that the thousandths digit could be estimated. The 3 isnot significant and would not be reported.rulerWhen you look at a reported measurement, it is necessary to be able to count the number of significantfigures. The table below details the rules for determining the number of significant figures in a reportedmeasurement. For the examples in the table below, assume that the quantities are correctly reported valuesof a measured quantity.Significant Figure RulesRuleExamples1. All nonzero digits in a measurement aresignificantA. 237 has three significant figures.B. 1.897 has four significant figures.2. Zeroes that appear between other nonzerodigits are always significant.A. 39,004 has five significant figures.B. 5.02 has three significant figures3. Zeroes that appear in front of all of thenonzero digits are called left-endzeroes. Left-end zeroes are never significantA. 0.008 has one significant figure.B. 0.000416 has three significantfigures.4. Zeroes that appear after all nonzero digitsare called right-end zeroes. Right-endzeroes in a number that lacks a decimal pointare not significant.A. 140 has two significant figures.B. 75,210 has four significant figures.5. Right-end zeroes in a number with adecimal point are significant. This is truewhether the zeroes occur before or after thedecimal point.A. 620.0 has four significant figures.B. 19.000 has five significant figures11
Uncertainty in MeasurementIt needs to be emphasized that to say a certain digit is not significant does not mean that it is not important orcan be left out. Though the zero in a measurement of 140 may not be significant, the value cannot simply bereported as 14. An insignificant zero functions as a placeholder for the decimal point. When numbers arewritten in scientific notation, this becomes more apparent. The measurement 140 can be written as 1.4 102 with two significant figures in the coefficient. For a number with left-end zeroes, such as 0.000416, it canbe written as 4.16 10 4 with 3 significant figures. In some cases, scientific notation is the only way tocorrectly indicate the correct number of significant figures. In order to report a value of 15,000,000 with foursignificant figures, it would need to be written as 1.500 107. The right-end zeroes after the 5 are significant.The original number of 15,000,000 only has two significant figures.Adding and Subtraction Significant FiguresFor addition and subtraction, look at the decimal portion (i.e., to the right of the decimal point) of the numbersONLY. Here is what to do:1. Count the number of significant figures in the decimal portion of each number in the problem. (Thedigits to the left of the decimal place are not used to determine the number of decimal places in thefinal answer.)2. Add or subtract in the normal fashion.3. Round the answer to the LEAST number of places in the decimal portion of any number in theproblem.Multiplying and Dividing Significant FiguresThe following rule applies for multiplication and division:1. The LEAST number of significant figures in any number of the problem determines the number ofsignificant figures in the answer.2. This means you MUST know how to recognize significant figures in order to use this rule.12
Uncertainty in MeasurementDensityOne of the ways a scientist identifies a substance is by calculating its density. Density is defined as the mass of an object in a givenunit of volume. This means that the property of density tells how tightly matter is packed in a substance. You have probably heardof the famous riddle, “Which weighs more, a pound of feathers or a pound of lead?” At first many people say, a pound of lead. Butthe answer is that they weigh the same (one pound each). This riddle illustrates the physical property of matter called density. Thedensity of lead is much greater than the density of feathers. A pound of lead would be a small cube, while a pound of featherswould be in a much larger box. Another way of saying this is that lead has more matter packed in a smaller space than the feathershave.What is Density?The idea of density can be expressed in mathematical terms. The formula for density is: density mass divided by volume (d m/v). So, density is found by dividing mass of an object by its volume. The units for density are usually grams per cubic centimeter(g/cm3) or grams per milliliter (g/mL). Mass is the amount of matter in an object, measured with a balance in the base unit ofgrams; however volume is the amount of space an object occupies and is measured in the liquid base unit as milliliters (mL) andsolid base unit of cubic centimes (cm3) . One milliliter is equal to one cubic centimeter. For example, the mass of an object is 30grams and its volume is 15 milliliters. We find the density of the object by dividing 15 into 30. The density would be recorded as 2grams per milliliters or 2 g/mL.Here is the same example using the proper set up and math:D mVD ?m 30 gV 15 mLD 30 g .15mLD
Metric Prefixes Conversions between metric system units are straightforward because the system is based on powers of ten. For example, meters, centimeters, and millimeters are all metric units of length. There are 10 millimeters in 1 centimeter and 100 centimeters in 1 meter. Metric prefixes are used to distinguish between units of different size.
Name Symbol Atomic Name Symbol Atomic . Number Number Actinium Ac 89 Mercury Hg 8o Aluminum Al 13 Molybdenum Mo 42 Americium Am 95 Neodymium Nd 6o . Einsteinium Es 99 Scandium Sc 21 Erbium Er 68 Selenium Se 34 Europium Eu 63 Silicon Si 14
atomic mass or atomic number the exercise is focused on alpha and beta emissions. Key Alpha particle: Beta particle: Gamma ray: In the example, Rn is the atomic symbol for the element Radon. The number 222 indicates the atomic mass of the element (or isotope). The number 86 respresents the element’s
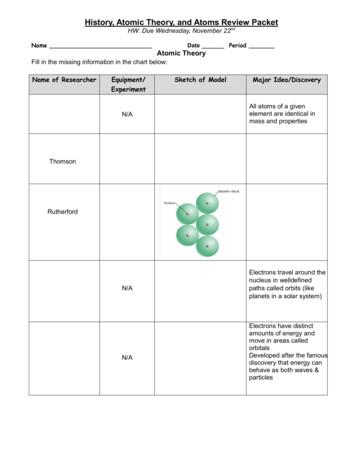
Atomic Structure Early Atomic Theories Dalton’s Atomic Theory: Each element is composed of extremely small particles called atoms. All of the atoms of 1 element are identical to each other but are different from the atoms of another element. Atoms of one element cannot be changed into atoms of a different element by chemical
atomic number 11 In the isotope symbol, the atomic number is written as a subscript (bottom left). Please note that in the periodic table, the atomic number is most often written above the symbol. In order to avoid making a mistake, please use the whole number (not the number with decimal
that particular element. EACH ELEMENT HAS ITS OWN DISTINCT ATOMIC NUMBER. 2. ATOMIC SYMBOL: is a one, two or three letter “code” or abbreviation that represents that particular element. 3. ATOMIC MASS: indicates the AVERAGE NUMBER OF PROTONS NEUTRONS in the nucleus of each isotope of that particular element.
Example Exercise 9.1 Atomic Mass and Avogadro's Number. The atomic mass of each element is listed below the symbol of the element in the periodic table: Cu 63.55 amu, Hg 200.59 amu, S 32.07 amu, and He 4.00 amu. The mass of Avogadro's number of atoms is the atomic mass expressed in grams. Therefore, 6.02 . . 10. 23. atoms of
Lines 20-21: Atomic species declaration After the keyword ATOMIC_SPECIES, for each ntyp enter atomic symbol atomic weight pseudo-potential Lines 22-24: Atomic positions After the keyword ATOMIC_POSITIONS, for each nat enter atomic symbol x y z where x,y,z are given as fractional coordinates
Atomic Structure Worksheet **Assume all are neutral atoms! Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number Protons Neutrons Electrons Mass number