Dept Of Chem., B.Sc (Chemistry)-Re Str. Syllabus Effective .
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTSSEMESTERCourseMCIAR-1 To Chem DeptAR-1 To other Depts*MCIIAR-2 To Chem DeptAR-2 To other Depts*IIIMCAO-1 To other Depts*EG-1 To other Depts*MCIVAO-2 To Other DeptsEG-2 To other Depts*MCVES(Any two)MSPackage-A or BVISKCH-6650 or -6617CH-6650CH-6651CH-6652Title of the Paperh/CrPageAnalytical ChemistryBasic Concepts in Inorg.ChemistryVol Anal &Inorg. Prep PracticalsMaths for ChemistryChemistry for Biologists-IChemistry for Biologist practicals-IChemistry of hydrocarbonsThermodynamicsOrganic qualitative analysisPhysics for ChemistryPhysics for Chemistry practicalsGeneral Chem for Maths& PhysicsGeneral Chem for Maths& Physics PracticalsOrganic functional groupsMain group elements & Solid state chemistryInorganic qualitative analysisChemistry for biologists-IIChemistry practical for biologists-IIChemistry in everyday lifeElectrochemistryPhysical chemistry practicalsComputer applications for chemistryGen Chemistry for Maths&Phys-IIGen Chemistry Lab for Maths&Phys-IIBasic clinical & pharmaceutical chemistryOrgano-Nitrogen compounds & stereochemistryTrans elem& nuclear chemistryPhase equilibria& kineticsFundamentals of spectroscopyGrav. Anal & Org. preparationsPolymer chemistry (or)Forensic chemistryBiochemistry (or)Agricultural chemistryMolecular dynamicsCoordination chemistryChemistry of materials (Pack-A)Synthetic organic chemistry (Pack-A)Chemistry of Natural products (Pack-B)Industrial chemistry (Pack-B)Food chemistry Food Chem.practical Industrial exposureChem. of consumer products Chem of consumerproducts practicals Industrial exposureGen trends in Industrial Chem (Seminar & 952535456576062646769717375TOTAL h/Cr (To ChemDept)TOTAL h/Cr (To other 148085
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTSSubjectAllied requiredAR1.5h 1 CrSem-I h/cMT-1102 :Math.forChem6/4Sem-IIh/cSem-III h/cSem-IV h/cSem-VPH-2105: Phys forChem4 /3PH-2106: Phys forChem (Pract) 2/1Allied optional# One AO from other#One AO from otherAODept.(AO-1)Dept.(AO-2)6/41.5hrs 1 Cr{Maths, PB or Zoo)6/4{Phys, PB or Zoo)CH-5402 PolymCElective SubjectES (Two)1.5h 1 CrCH-5403 ForensCH-5404 BiocheCH-5405 Agri.ChMajor CoreCH-1505:CH-2506: HydrocarbonsCH-3506: Organic Fn.CH-4504: ElectroCH-5510:OrgNitMCAnal Chem. 3/33/3Gp3/3chem. 3/3&Stereo5/5CH-1506: Conceptsin Inorg. Chem.CH-2507 :CH-3507: Main Gp&CH-4505:Phy.ChemCH-5511:TransEThermodynamics 3/3Solid State Chem.Pract3/3Nucl. Chem5/5CH-2508 :Org.Qual.Analysis3/3CH-4506: Comp.CH-5512: PhaseCH-3508 :Inorg. Qual.Appl. for Chem3/3and Kinetics 5/51 h 1 Cr3/3CH-1507: Vol. Anal.Inorg. Prepn.Pract3/33/3AnalCH-5513: Fund.3/3CH-5514:Gra.AnOrg.Preps4/4*Major SpecialMSPackage-A or B
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTS1.5 h 2 Cr*Skill BasedSK1 h 1 CrTotal15/1315/1315/1315/13#Courses offered to other Departments:AR-1:CH-1100,1101:Chem for biologists – I (Sem- I)3301:Chem in Everyday Life (Sem-III) 3h/1cAO-1: CH-3204, 3205:Biochem for Biologists – II (Sem-III)EG-1:CH-AR-2:CH-2104,2105: Gen chem for Maths& Physics – I (Sem- II)AO-2: CH-4206,4207:General chem for Maths and Physics – II(Sem-IV)EG-2: CH-4302:Basic Cl&Pharm.Chem (Sem-IV) 3h/1cDepartment of ChemistryRESTRUCTURED SYLLABUSB.Sc. (Chemistry)(Effective from 2012-2013)I-SEMESTERCH-1505: ANALYTICAL CHEMISTRYSemester – INo. of credits: 3Course: Major Core (MC)No. of hours:3 h/wk30/28
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTSObjectives:1.To enable the student to develop the habit of handling analytical data.2.To learn the principles of basic analytical methods and their applications.Unit-1: Handling of Chemicals and Data analysis(10 h)1.1. Safety and hygiene in the Chemistry LabStorage and handling of chemicals, Handling of acids, ethers, toxic and poisonouschemicals.Antidotes, threshold vapour concentration and first aid procedure. MSDS,COSHH.1.2. Error in chemical analysisAccuracy and precision, Absolute and relative errors.Methods of eliminating or minimizingerrors. Precision: mean, median, average deviation and coefficient of variation. Significantfigure and its relevance.Normal error curve and its importance.Unit-2: Separation and Purification Techniques(10 h)2.1. Chromatographic techniques and matography:ColumnandPaperchromotography. TLC, ion-exchange chromatography - technique and applications.Gaschromatography, principle, detector and applications.2.2. General purification techniquesPurification of solid organic compounds: recrystallisation, sublimation. Use of misciblesolvents.Use of drying agents and their properties.Purification of liquids.Techniques ofdistillation.Chemical methods of purification and test of purity.Unit-3: Titrimetric Methods of Analysis(10 h)3.1. General PrincipleMethods of expressing concentration of solutions.Types of titrations.Requirements fortitrimetric analysis.Primary and secondary standards.Limitation of volumetric analysis.3.2. Acid-base EquilibriapH of strong and weak acid solutions. Buffer solutions.Henderson equations.Preparation ofacidic and basic buffers.Relative strength of acids and bases from K a and Kbvalues.Neutralisation-titration curve, theory and choice of indicators.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTS3.3. Complexometric titrationsStability of complexes.Titration involving EDTA. Metal ion indicators and theircharacteristics.Unit-4: Solubility Equillibria(10 h)4.1. Precipitation c titrations, indicators for precipitation titrations involving silvernitrate.Determination of chloride by Volhard’s method.Adsorption indicators.4.2. Gravimetric methods of analysis:Separation by precipitation.Factors affecting solubility, gravimetric factor.Purity ofprecipitates, von Weiman ratio. Co-precipitation and post precipitation. Precipitation fromhomogeneous solution.Unit-5: Thermal Analysis(5 h)5.1. Principle of thermogravimetric analysis (TGA). Differential thermal analysis (DTA):Instrumentation and applications.5.2. Factors affecting TGA and DTA curves. TGA of AgNO3, CaC2O4.H2O and DTA ofsulphur.Text Books :1.R.A. Day and A.L. Underwood, Quantitative Analysis, 5th ed., Prentice Hall of IndiaPrivate Ltd., New Delhi, 1988.2.U. N. Dash, Analytical Chemistry: Theory and Practice, Sultan Chand and sonsEducational Publishers, New Delhi, 2011.3.B. R. Puri and L.R. Sharma, Principles of Physical Chemistry, Shoban Lal Nagin Chandand Co. 33rd ed., 1992.4.R. Gopalan, P. S. Subramanian and K. Rengarajan, Elements of Analytical Chemistry,Sultan Chand, New Delhi, 2007.5.S. Usharani, Analytical Chemistry, McMillan Publisher, 2000.References :1.D. A. Skoog, D. M. West and F. J. Holler, Analytical Chemistry: An Introduction, 5thed., Saunders college publishing, Philadelphia, 1998.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTS2.H. Kaur Instrumental Methods of Chemical Analysis Pragati Prakashan, Meerut, 2010.3.V.K. Srivastava, K.K. Srivastava, Introduction to Chromatography: Theory and Practice,S. Chand and Company, New Delhi, 1987.4.A.K. Srivastava, P.C. Jain, Chemical Analysis: An Instrumental Approach for B.Sc. Hons.and M.Sc. Classes, S. Chand and Company Ltd., Ram Nagar, New Delhi, 2010.CH-1506: BASIC CONCEPTS IN INORGANIC CHEMISTRYSemester-INo. of credits: 3Course: Major Core (MC)No. of hours: 3h/wkObjectives :1. To know the arrangement of elements in the periodic table and the periodic properties.2. To understand the different kinds of chemical forces in molecules.3. To identify the nature of chemical bond in a given inorganic compound.Unit 1: Atomic Structure and Periodic Table1.1(10 h)Electronic configuration: Bohr theory, duel nature of electrons, Heisenberg uncertaintyprinciple, Pauli’s exclusion principle, Hund’s rule, sequence of energy levels (Aufbauprinciple).1.2Periodicity: Periodic law and arrangement of elements in the periodic table, IUPACnomenclature and group number. Horizontal, vertical and diagonal relationships in theperiodic table.1.3Properties ofatoms: Size of atoms and ions-atomic radii, ionic radii, covalent radii; trendin ionic radii, ionization potential, electron affinity; electronegativity-Pauling, MullikenJaffe, Allred-Rochow definitions; oxidation states and variable valency; isoelectronicrelationship; inert-pair effect. Atomic, molecular and equivalent weights; Avagadro’sprinciple and mass-volume relationship.Unit 2: Ionic bond(10 h)2.1 Properties of ionic compounds, factors favoring the formation of ionic compoundsionization potential, electron affinity, and electronegativity.2.2 Lattice energy(Uo) : Born-Lande equation (derivation not required). Factors affecting latticeenergy. Born-Haber cycle-enthalpy of formation ( Hf ) of ionic compounds.Stability andsolubility of ionic compounds of alkali- and alkaline earth metals on the basis of Hf andUo. Enthalpy of salvation and enthalpy of solution.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTS2.3 Covalent character of ionic compounds-Fajan’s rules; effects of polarization-solubility,melting points, and thermal stability of typical ionic compounds.Unit 3: Covalent Bond(12 h)3.1 Lewis theory-Octet rule and its exception, electron dot structural formula; Sidgwick-Powelltheory-prediction of molecular shapes; Valance Bond theory-arrangement of electrons inmolecules. Hybridization and geometry of molecules.3.2 VSEPR model-Effect of bonding and nonbonding electrons on the structure of molecules,effect of electronegativity. Illustration of structures by VESPR model-NH3, SF4, ICl4-, ICl2-,XeF4, XeF6.3.3 MO theory: LCAO method-criteria of orbital overlap, types of molecular orbitals- , - and -MOs; combination of atomic orbitals to give - and -MOs and their schematicillustration; qualitative MO energy level diagram of homodiatomic molecules-H2 to Ne2 andtheir magnetic properties, bond order and stability of molecules.Unit 4: Metallic and Weak Bonds(5 h)4.1 Metallic bond: Metallic properties, band theory of metals; semiconductors: n- and p-typesemiconductors.4.2 Weak bonds: Hydrogen bonding-intra- and intermolecular hydrogen bonding, influence onthe physical properties of molecules, comparison of hydrogen bond strength and propertiesof hydrogen bonded N, O, and F compounds; crystalline hydrates and clathrates; van derwaals forces, ion dipole-dipole interactions.Self-study: Properties of molecules exhibiting inter- and intramolecular hydrogen bonding.Compounds formed by London dispersive forces and van der Waals forces.Unit 5: Acids and Bases(8 h)5.1 Types of chemical reactions: Acid-base, oxidation-reduction, electron transfer and doubledecomposition reactions. Balancing chemical reactions by oxidation number and ionelectron method.5.2 Theories of acids and bases: Arrhenius theory of acids and bases in protic solvents.Bronsted-Lowry theory, Lewis theory, the solvent system.Lux-Flood definition andUsanovich definition.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTS5.3 Nonaqueous solvents: Classification-protonic and aprotic solvents. Liquid ammonia assolvent-solutions of alkali and alkaline earth metals in ammonia.Self-study: Common protic and aprotic nonaqueous solvents.Identification of acids and basesaccording to different definitions.Text Books:1.J. D. Lee, Concise Inorganic Chemistry, 5th ed., Blackwell Science, London, 1996.2.F. A. Cotton, G. Wilkinson and P. L. Guas, Basic Inorganic Chemistry, 3rd ed., John Wiley, 1994.3.B. Douglas, D. McDaniel and J. Alexander, Concepts and Models of Inorganic Chemistry,3rd ed., John Wiley, 1994.4.B. R. Puri, L. R. Sharma, K. C. Kalia, Principles of Inorganic Chemistry, Shoban Lal NaginChand and Co., 1996.References:1.J. E. Huheey, E. A. Kieter and R. L. Keiter, Inorganic Chemistry, 4th ed., Harper Collins,New York, 1993.2.D. F. Shriver and P. W. Atkins, Inorganic Chemistry, 3rd ed., W. H. Freeman and Co, London, 1999.3.T. Moeller, Inorganic Chemistry: A Modern Introduction, Wiley, New York, 1990.CH-1507: VOLUMETRIC ANALYSIS AND INORGANIC PREPARATIONSSemester – INo. of credits: 3Course: Major Core (MC)No. of hours : 3h/wkObjectiveTo enable the students to acquire the quantitative skills in volumetric analysis.A. Volumetric Practicals1.Calibration of volumetric apparatus: Burette, pipette and standard flasks.2.Acid – base titrations:a. Estimation of HCl.b. Estimation of oxalic acid.3.Redox titrations:a. Estimation of Ferrous ammonium sulphates (Permanganometry).b. Estimation of calcium (Permanganometry).c. Estimation of KMnO4 (Iodometry).
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTSd. Estimation of copper (Iodometry).e. Estimation of Fe2 -Fe3 mixture using diphenyl amine (Dichrometry)3.Complexometric titrations:a. Estimation of calcium.b. Estimation of magnesium.B. Inorganic preparationsa. Preparation of Ferrous ammonium sulphate.b. Preparation of tetraamminecopper(II) sulphate.c. Preparation of potassium trioxalatoaluminate.d. Preparation of potassium trioxalatochromateText books:S. Sundaram and K. Raghavan, Practical Chemistry , S. Viswanathan Co. Pvt., 1996.N. S. Ganapragasam and G. Ramamurthy, Organic Chemistry – Lab manual, S.Viswanathan Co. Pvt., 2002.Reference:1. B.S. Furniss, A.J. Hannaford, P.W. G. Smith and A.R. Tatchell, Vogel’s Text Book ofPractical Organic Chemistry.5th ed., Pearson Education, 2005.1.2.MT 1102: MATHEMATICS FOR CHEMISTRY -ISemester – INo. of credits: 4Course: Allied Required (AR-1)No. of hours: 6h/wkObjective:To familiarize the applications of mathematics to chemistry.Unit-1: Differentiation of standard functions-hyperbolic and inverse hyperbolic functionsdifferentiation of one function with respect to another-slope-tangent and normal-maxima andminima-angle of intersection of curves in cartesian and polar coordinatesUnit-2: Methods of integration-integration by parts-Bernoulli’s formula-properties of definiteintegrals-differential equations-second order differential equations with constant coefficients.Unit-3: Application of binomial, exponential and logarithmic series to summation-eigenvaluesand eigenvectors (differential calculus approach)-partial differential equations-all types.Unit-4: Complex numbers- DeMoivre’s theorem and applications-expansions of sin n , cos n ,sinn , cosn , sin , cos -hyperbolic functions-Fourier series.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTSUnit-5: Probability-mean-standard deviation-Binomial, Poisson and normal distributions.Text ,Pillai,Vol.IandVol.II,S.Viswanathan Printers & Publishers, 1996.2.T.K.Manickavachagam Pillai, T.Natarajan. andK.S. Ganapathy, Algebra, Vol I,S.Viswanathan Printers & Publishers,1994.3.S.Narayanan, Trigonometry, S.Viswanathan Printers & Publishers, 1995.4.S.P. Gupta, Elements of Statistics, S.Chand & Co, 1986.5.M.K.Venkataraman, Engineering Mathematics, III-A, The National Publishing Co.,1995.References:1.N.Shanthi ,Differential Calculus, S.Chand & Co., 1964.2.P.R.Vittal,.Trigonometry, Margham Publications,1988.3.P.Duraipandian, Vector Analysis, Emerald Publishers, 1984.CH-1100: CHEMISTRY FOR BIOLOGISTS-I(Offered to B.Sc. Zoology and Plant Biology)Semester – INo. of credits: 3Course: Allied Required (AR-1)No. of hours:4h/wkObjective:To enable the students to understand the concepts of chemistry.Unit 1: Handling of chemicals and Data analysis1.1(15 h)Storage and handling of chemicals: Handling of acids, ethers, toxic and poisonouschemicals. Antidotes, threshold vapour concentration and first aid procedure.1.2Errors in chemical analysis: Accuracy, precision. Types of error-absolute and relativeerrors. Methods of eliminating and minimizing errors.1.3Separation techniques–Solvent extraction. Principle of adsorption and partitionchromatography, column chromatography, thin layer chromatography (TLC), paperchromatography and their applications.Unit 2: Chemical bonding2.1(15 h)Ionic Bond: Nature of Ionic bond. Structure of NaCl, KCl and CsCl. Factors influencing theformation of ionic bond.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTS2.2Covalent Bond: Nature of covalent bond. Structure of CH4, NH3, H2O based onhybridisation.2.3Coordinate Bond: Nature of coordinate bond. Coordination complexes.Werner’stheory.Geometrical and optical isomerism in square planar and octahedral complexes.Mention of structure and functions of chlorophyll and hemoglobin2.4Hydrogen Bond: Theory and importance of hydrogen bonding. Types of hydrogenbonding.Hydrogen bonding in carboxylic acids, alcohol, amides, polyamides, DNA andRNA.2.5van der Waal’s forces: Dipole – dipole and dipole - induced dipole interactions.Unit 3: Volumetric analysis(10 h)3.1Methods of expressing concentration: normality, molarity, molality, ppm.3.2Primary and secondary standards: preparation of standard solutions3.3Principle of volumetric analysis: end point and equivalence points.3.4Strong and weak acids and bases - Ionic product of water , pH, pKa, pKb. Buffer solutions pH of buffer solutions. Mention of Henderson equation & its significance.Unit 4: Kinetics4.1(10 h)Chemical Kinetics: Rate, rate law, order and molecularity. Derivation of rate expressionsfor I and II order reactions.4.2Catalysis-Homogeneous and heterogeneous catalysis. Enzyme catalysis, enzymes inbiological system and in industry.Unit 5: Chemistry of biomolecules5.1Fats – Occurrence and composition. Hydrolysis of fats.5.2Vitamins – Source, provitamin, properties and classification. Structure and function(10 h)of vitamin A, C, D, K and E5.3Hormones – Thyroxin, adrenaline and sex hormones (structure and functions only)Text Books:1. R. Gopalan, S. Sundaram, Allied Chemistry, Sultan Chand and Sons, 1995.2.U. Sathyanarayana, Biochemistry, Books and allied (p) Ltd, 1999.3.B.R.Puri and L.R.Sharma, Principles of physical chemistry, Shoban Lal Nagin Chand andCo. 33rd ed., 1992.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTSReferences:1.D.A. Skoog, D.M. West and F.J. Holler, Analytical Chemistry: An Introduction, 5thed., Saunders college publishing, Philadelphia, 1990.2.G.C. Hill, J.S. Holman, Chemistry in Context, ELBS, 19983.W.R. Kneen, M.J.W. Rogers, P. Simpson, Chemistry – Facts, patterns and principles,ELBS, 1999.CH-1101: CHEMISTRY PRACTICAL FOR BIOLOGISTS-I(Offered to B.Sc. Zoology and Plant Biology)Semester – ICredit: 1Course: Allied Required(AR-1)No. of hours :2h/wkObjective:To enable the students to understand the concept of organic analysis.Organic Analysis:a)Detection of N, S and halogensb)Test for aliphatic and aromatic nature.c)Test for saturation and unsaturation.d)Nature and identification of the following functional groupsi) Carboxylic acidii) Phenolsiii) Aldehydesiv) Ketonesv) Carbohydratesvi) Primary aminesvii) AmidesText Books:1.N.S. Gnanapragasam and G. Ramamurthy, Organic chemistry – Lab manual, S.Viswanathan Co. Pvt. Ltd., 2002.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective from 2012-2013-CONTENTS2.J.N. Gurtu and R. Kapoor, Advanced Experimental Chemistry (Organic), S. Chand andCo., 1987.
Dept of Chem., B.Sc (Chemistry)-Re Str. Syllabus effective f
CH-6650 Food chemistry Food Chem.practical Industrial exposure 13/13 75 CH-6651 Chem. of consumer products Chem of consumer products practicals Industrial exposure 13/13 80 CH-6652 Gen trends in Industrial Chem (Se minar & Report) 2/2 85 TOTAL h/Cr (To ChemDept) 120/131 TOTAL h/Cr (To other Dept*) 24/14
CHEM 350B Topics in Chemistry 7.5 454.95 CHEM 351 Chemicals Big and Small: Nano- 15 909.90 CHEM 352 Advanced Concepts in Chemistry 15 909.90 CHEM 352A Advanced Concepts in Chemistry 7.5 454.95 CHEM 352B Advanced Concepts in Chemistry 7.5 454.95 CHEM 360 Contemporary Green Chemistry 15 909.90 CHEM 380 Materials Chemistry 15 909.90
CHEM 31X. Chemical Principles 4 CHEM 33. Structure and Reactivity 4 CHEM 35. Organic Monofunctional Compounds 4 CHEM 36. Organic Chemistry Laboratory I 3 MATH 41, 42, 51. Calculus, Linear Equations 5 5 5 SECOND YEAR CHEM 130. Organic Chemistry Laboratory II 4 CHEM 131. Organic Polyfunctional Compounds y3 CHEM 134.
CHEM 110 Chemistry of the Living World 15 4,736.85 CHEM 120 Chemistry of Material World 15 4,736.85 CHEM 150 Concepts in Chemistry 15 4,736.85 CHEM 200 Special Topic 15 4,736.85 CHEM 251 Structure and Spectroscopy 15 4,736.85 CHEM 252 Properties and Analysis of Mat 15 4,736.85
WRF-Chem Version 3.9.1.1 User’s Guide Table of Contents 1.1 WRF-Chem Introduction3 1.2 WRF-Chem software 5 1.3 Possible applications of the current modeling system 5 1.4 The WRF-Chem modeling system overview 5 2.1 Software Installation Introduction 8 2.2 Building the WRF-Chem code 9 2.2.1 Getting the code 9
bonding and reactions) necessary for courses in elementary organic chemistry and physiological chemistry. Students may only receive credit toward graduation for one of the following: CHEM 10050; or CHEM 10060 and CHEM 10061; or CHEM 10970 and CHEM 10971.
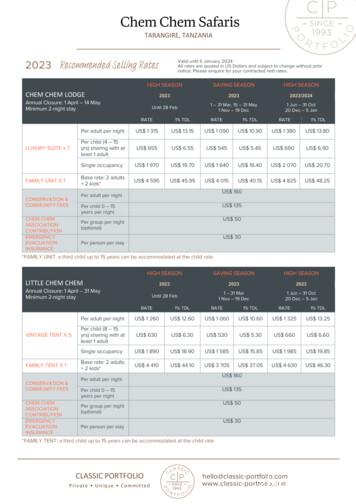
Chem Chem Safaris TARANGIRE, TANZANIA Updated: 9 November 2022 Page: 2 of 6 www.classic-portfolio.com CLASSIC PORTFOLIO Private Unique Committed hello@classic-portfolio.com www.classic-portfolio.com FOREST CHEM CHEM Annual Closure: 1 April - 31 May Minimum 2-night stay SAVING SEASON HIGH SEASON 2023 2023/2024 1 - 31 Mar 1 Nov - 19 Dec
Apr 11, 2007 · State Dept. of Education Dept of Children and Families Judicial Department Higher Education Community and Technical colleges Public Health Dept of Mental Health and Addiction Services Dept of Mental Retardation Dept t of Labor Dept of Transportation Dept of Corrections We do not have a
Studying astrology can evoke changes in how we see life and experience the world, and in our lives, and for this reason it is important that students take their time with their studies and view study as a journey rather than a destination. There are times where there is greater studying activity and other times of greater reflection or adjustment, both of which are of immense value. It is .