CHEMISTRY REVISION GUIDE For CIE IGCSE Coordinated Science .
CHEMISTRY REVISION GUIDEfor CIE IGCSE Coordinated Science (2012 Syllabus)This revision guide is designed to help you study for thechemistry part of the IGCSE Coordinated Science course.The guide contains everything that the syllabus says youneed you need to know, and nothing extra.The material that is in the supplementary part of thecourse (which can be ignored by core candidates) ishighlighted in dashed boxes:Whilst this guide is intended to help with your revision, itshould not be your only revision. It is intended as astarting point but only a starting point. You should makesure that you also read your text books and use theinternet to supplement your study in conjunction withyour syllabus document.Whilst this guide does contain the entire syllabus, it justhas the bare minimum and is not in itself sufficient forthose candidates aiming for the highest grades. If that isyou, you should make sure you read around a range ofsources to get a deeper knowledge and understanding.Some very useful websites to help you further yourunderstanding include: http://www.docbrown.info/ - whilst not theprettiest site this contains a lot of very useful andnicely explained information. http://www.bbc.co.uk/schools/gcsebitesize/science/ - well presented with many clear diagrams,animations and quizzes. Can occasionally lackdepth. http://www.chemguide.co.uk/ - whilst mostlytargeted at A-Levels this site contains very detailedinformation suitable for those looking to deepentheir knowledge and hit the highest grades.Finally, remember revision is not just reading but shouldbe an active process and could involve: Making notes Condensing class notes Drawing Mind-maps Practicing past exam questions Making flashcardsThe golden rule is that what makes you think makes youlearn.Happy studying, Mr Field.
C1: THE PARTICULATE NATURE OFMATTERAtom: The smallest particle An atom:of matterSome atoms:Molecule: A small particlemade from more than oneatom bonded togetherMolecules of an element: Molecules of acompound:Element: A substancemade of only one type ofatomA solid element:Compound: A substanceA solid compoundmade from two or moredifferent elements bondedtogetherMixture: A substancemade from two or moreelements or compoundsmixed but not joinedA gaseous element:A gaseous compound:A mixture of compounds and elements:Solids, Liquids andGasesSOLIDS LIQUIDS AND GASESThe particles in solids, liquids and gases are held near to each other by forces ofattraction. The strength of these forces determines a substance’s melting andboiling points.In a solid, the forces of attraction are strongest, holding the particles tightly inposition. As the solid is heated, and the particles vibrate faster, these forces arepartially overcome allowing the particles to move freely as a liquid – this is calledmelting. As the liquid is heated more, the particles gain so much energy that theforces of attraction break completely allowing particles to ‘fly around’ as a gas –this is called boiling. The reverse of the these processes are condensing andfreezing. Under specific conditions, some solids can turn straight to gases – aprocess called subliming (the reverse is called desubliming).PROPERTIESSolids Have a fixed shape Can’t be compressed Particles close togetherin a regular pattern Particles vibrate arounda fixed pointLiquids Take the shape of theircontainer Can’t be compressed Particles close togetherbut disordered Particles move freelyGases Take the shape of theircontainer Can be compressed Particles widely spacedin random order Particles moving veryfast.
C2: EXPERIMENTALTECHNIQUESFILTRATIONUsed to separate solidsfrom liquids. The mixtureis poured through a filterpaper in a funnel. Theliquid can pass throughthe small holes in thefilter paper (to becomethe filtrate) and the solidgets left behind(called the residue).CRYSTALLISATIONCrystallisation is used to separate mixtures of solid dissolved inliquid and relies on the fact that solids are more soluble athigher temperatures. A solution containing a solid is cooleddown until crystals form in the solution, these can then becollected by filtration.The related technique of recrystallisation can be used toseparate a mixture of two soluble solids by taking advantage ofthe difference in their solubility. The mixture is dissolved in thesmallest possible amount of hot solvent. As the solution cools,the less soluble compound forms crystals that can be collectedby filtration whilst the more soluble compound stays dissolved.DISTILLATIONIn distillation a mixture ofliquids is separated using thedifferences in their boilingpoints. The mixture is heateduntil the liquid with the lowestboiling point boils, the vapoursthen condense on the coldsurface of the condenser andthe pure(er) liquid is collected.best possible separation of spots.PAPER CHROMATOGRAPHYPaper chromatography is a technique that can beused to separate mixtures of dyes or pigments andis used to test the purity of a mixture or to seewhat it contains. Firstly a very strong solution ofthe mixture is prepared which is used to build up asmall intense spot on a piece of absorbent paper.This is then placed in a jar of solvent (with a lid). Asthe solvent soaks up the paper, it dissolves themixture-spot, causing it to move up the paper withthe solvent. However since compounds havedifferent levels of solubility, they move up thepaper at different speeds causing the individualcomponents to separate out. The solvent orcombination of solvents can be changed to get thePURITYIt is important for chemists to be able to purify thecompounds they make, this is because theimpurities could be dangerous or just un-useful.This is especially true for chemists makingcompounds that are consumed by people such asdrugs or food additives since the impurities may betoxic which would be very bad news!WHICH TECHNIQUE?You need to be able to select appropriate methodsto separate a given mixture. The key to this is lookfor differences in the properties of thecomponents of the mixture such as their state,solubility, melting/boiling point and so on. Thenpick the method that best takes advantage of thisdifference.MELTING/BOILING POINTSNo two substances have the exact same meltingand boiling points. We can take advantage of thisto test the purity of a compound we have made. Ifwe know what the melting or boiling point of thepure compound should be, we can then measurethe melting or boiling point of a sample we haveproduced and the closer it is to the pure value, themore pure it is likely to be.FRACTIONAL DISTILLATIONWhen the liquids being distilled have similarboiling points, normal distillation can’t separatethem completely but simply gives a purermixture. In this case a fractionating column isused. This provides a large surface area forcondensation meaning much purer ‘fractions’ areproduced. The most important use of this isseparating crude oil into it’s useful components.
Example 2: Chlorine. Protonnumber is 17 which meansthere are 17 electrons: 2 inthe 1st shell, 8 in the secondand 7 in the 3rd.CClChecking Your Answer: To checkyou are right, the period gives thenumber of shells and the group gives the number of electronsin the outer shell. For example chlorine is in Period 3 andGroup VII so it has 3 shells and 7 electrons in the outer shell.Ions: The configuration of ions is the same as for atoms butyou have to take electrons away from positive ions and addextra for negative ions. For example O/O2- Li/Li OO2-LiLi For example if you melted somesolid sugar to a liquid and thenleft it to cool, it would freezeback to solid sugar – this is aphysical change. If you took thesame sugar and burned it toproduce carbon dioxide andwater, there would be no easyway to turn those back to sugar –this is a chemical change – newsubstances are made.ATOMIC STRUCTUREAtoms are made of:Protons: mass 1, charge 1Neutrons: mass 1, charge 0Electrons: mass 0, charge -1Transition MetalsOtherMetalsGroup VIII: Noble Gases Example 1: Carbon. Protonnumber is 6 which meansthere are 6 electrons: 2 in the1st shell and 4 in the secondCHEMICAL VS PHYSICALCHANGESPhysical changes are reversiblewhereas chemical changes arenot.Non-metalsGroup VII: HalogensThe number of electrons around an atom is given by theatom’s proton number. They are arranged in shells as follows: 1st Shell – Holds two electrons 2nd/3rd/4th Shells – Hold 8 electronsOther elements tend toreact in such a way as toachieve a full outer shell bygaining or losing electronsuntil they achieve this NobleGas configuration.HGroup II: Alkali-EarthELECTRON ARRANGEMENT/CONFIGURATIONElectrons are arranged around atoms in specific shells. Themost important shell is the outer one as this controls anatom’s chemistry. We call the electrons in the outer shell‘valence electrons’ because they are used for bonding. Thenumber of electrons in the outer shell is the same anelement’s group number.A NOBLE MATTERThe Noble Gases (He, Ne, Aretc) have full outer shellscontaining either 2 or 8electrons. This is very stablewhich is why the Noble gasesare so unreactive.Group I: Alkali MetalsC3: ATOMS, ELEMENTS ANDCOMPOUNDS – Structures andBondingLanthanides and Actinides (metals)STRUCTURE OF THE PERIODIC TABLE (PTon last page!)Elements arranged in order of increasingproton number.Periods: The rows in the periodic table. For example Li, C and O are all in period 2.Groups: The columns in the PT. Use roman numbers: I, II, III, IV, V, VI, VII,VIII Eg. F, Cl, Br, I are all in Group VII Elements in the same group have similarproperties and react in similar ways: thehalogens all react in the same way withsodium to form sodium fluoride (NaF),sodium chloride (NaCl), sodium bromide(NaBr) and sodium iodide (NaI)what the element is.In a square on the periodic tablethe smaller number, the protonnumber, gives the number ofprotons or electrons and theThe numbers of each vary frombigger number, the nucleonelement to element but it is the number the number of protonsnumber of protons which decides and neutrons together.ISOTOPESIsotopes are atomswith the same protonnumber but differentnucleon number.For example carbonhas two mainisotopes – C-12 andC-13. Carbon has aproton number of 6so they both contain6 protons and 6electrons but C-12has 6 neutrons and C13 has 7.Eg 1: Boron has 5 protons,6 neutrons (ie 11-5) and 5electronsEg 2: Phosphorus has 15protons, 16 neutrons (ie31-16) and 15 electrons
The atoms in a molecule arejoined by strong covalentbonds. In a solid eachmolecule is held close to itsneighbour by weakintermolecular forces.When a substance melts, it isthese weak intermolecularforces that break NOT thestrong covalent bonds.Molecular compounds havelow melting points and arevolatile (evaporate easily) dueto the weak intermolecularforces, and insulate electricityas all electrons are stuck inbonds and so unable to move.Atoms will lose or gain electrons until they have a complete outer shell: elements in Groups I, II and IIIwill lose 1, 2 and 3 electrons respectively to form 1 , 2 and 3 ions. Atoms in Groups V, VI and VIIgain 3, 2 and 1 electrons to form 3-, 2- and 1- ions. In an ionic compound the number of positive andnegative and charges must cancel out to neutral.Example: NaF, sodium in Group I forms a 1 ion Example: Li2O, lithium in Group I forms a 1 ionand fluorine in group VII forms a 1- ion so onebut oxygen in Group VI forms a 2- ion so two Li Na is needed to balance out one Fare needed to balance out one O2-Na F-Li COVALENT BONDINGA covalent bond forms between two atoms and is the attractionof two atoms to a shared pair of electrons. Small groups ofcovalent bonded atoms can join together to form molecules.GIANT COVALENT LATTICESA crystal made of a repeatingpattern of atoms joined withcovalent bonds that repeatsmillions of times in alldirections.Diamond is made of carbonatoms arranged so that each Cis bonded in a pyramidarrangement to 4 others. Thismakes it very hard, ideal for usein industrial drills:Graphite: made of carbonatoms arranged in hexagonalsheets with long weak bondsbetween the sheets. Thismeans the sheets can easilyseparate making graphite agood lubricant:Silicon (IV) oxide (SiO2) has astructure with each Sijoined to 4 O and each Ojoined to 2 Si. It isthe main ingredientin glass.Group VIII: Noble GasesMOLECULESA molecule is a small particlemade from (usually) a fewnon-metal atoms bondedtogether.IONIC BONDINGAn ionic bond is the attraction between two oppositely charged ions. Cations (positive) are formedNon-metalswhen atoms (usually metals) lose electrons. Anions (negative) are formed when atoms(usually nonmetals) gain electrons.Group VII: HalogensC3: ATOMS, ELEMENTS ANDCOMPOUNDS – Bonding andStructureThe atoms share enough electrons to complete their outershells.Example: H O*, hydrogen isExample: CO *, carbon is has22has one valence electron andneeds one more to completethe 1st shell, oxygen has sixvalence electrons electrons soneeds two more. Thus oneoxygen will react with twohydrogens:HOHfour valence electrons soneeds four more to completeits outer shell, oxygen needstwo more. Thus each carbonwill react with two oxygens,sharing two electrons witheach one. A bond involvingtwo shared pairs is a doublebond.OCO*Nb: In these diagrams only draw the outer shell and usedifferent shapes/colours to show where electrons have comefrom. You should be able to draw at least: H2O, CH4, Cl2, HCl, H2,N2, O2, CO2, C2H4O2-Li GIANT IONIC LATTICESThe positive and negative ions inan ionic compound don’t formmolecules but form crystals madeof a repeating pattern of positiveand negative ions called a giantionic lattice. Eg sodium chloride:Properties of Ionic CompoundsWhen you melt or dissolve anionic compound it conductselectricity because the ions arefree to move towards the positiveand negative electrodes. Whensolid the ions are stuck in positionand there are no free electrons sothey don’t conduct.
C4: STOICHIOMETRY –Formulas and EquationsSYMBOL EQUATIONS Show the reactants you start with and the products youmake using symbols not words Must contain an arrow ( ) NOT an equals sign ( ) Must be balanced – same number of atoms on each side. Balancing is done by placing numbers called coefficients infront of the formulas for the compounds/elements. Forexample, ‘O2‘ means there is one oxygen molecule involved ina reaction but ‘2O2’ would mean there are two.Example:. CH4(g) O2(g) CO2)g) H2O(g)*This is unbalanced as there are 4 ‘H’ on the left but only 2 ‘H’on the right. This must be corrected by placing a ‘2’ in front ofthe ‘H2O’ so there are now 2 waters:CH4 (g) O2(g) CO2(g) 2H2O(g)Now the ‘H’ balances but there 4 ‘O’ on the right and only 2on the left. This must be balanced by placing a ‘2’ in front ofthe ‘O2’ so that there are 2 oxygen molecules:CH4(g) 2O2(g) CO2(g) 2H2O(g)Now there is 1 ‘C’, 4 ‘H’ and 4 ‘O’ on each side so it balances.In ionic equations, we tend to look only at the ions thatactually change. For example, when iron reacts with coppersulphate to form iron sulphate and copper the equation is:Fe(s) Cu2 (aq) SO42-(aq) Fe2 (aq) SO42-(aq) Cu(s)In this case, the sulphate ion (SO42-) remains unchanged (wecall it a spectator ion) so it can be left out of the equation togive:Fe(s) Cu2 (aq) Fe2 (aq) Cu(s)This allows us to see more clearly the actual chemical changestaking place.Note: You can’t change the little numbers (ie the 2 in H2O ) asthis changes the compound to something completelydifferent.*The state symbols (s), (l), (g) and (aq) are used to indicatesolid, liquid, gas and ‘aqueous solution’ (dissolved in water).CHEMICAL FORMULASFormulas tell you the atoms that make up acompoundEg 1. H2O – two H, one OEg 2. C2H6O – two C, six H, one OEg 3. Mg(OH)2 – one Mg, twoEg 4. CH2(CH3)2 – three C, 8 H*O, two H**In this case everything in brackets is doubledYou may be asked towrite a formula given adiagram of a moleculefor example glucose.By counting you can seethere are 6 carbons,12 hydrogens and6 oxygens so theformula is C6H12O6WORD EQUATIONS These tell you the names of the chemicals involved inreaction The left hand side shows you what you start with andis called the reactants The right hand side shows you what you make and iscalled the products The left and right are connected by an arrow ( not‘ ‘) which means ‘makes’ or ‘becomes’ When you react a metal with oxygen to make a metaloxide, the equation might be:Iron oxygen iron oxide Many fuels burn in oxygen to produce carbon dioxideand water for example:Methane oxygen carbon dioxide waterCHEMICAL MASSESThe relative atomic mass (Ar) of an element is themass of one atom relative to 1/12th the mass of C12. It is just a number that allows us to comparethe mass of atoms of different elements. Ar can befound on the periodic table as the ‘large’ numberin each square. For example Ar for carbon is 12.01and for iron is 55.85. Ar has no units since it is onlya relative number, allowing us to compare things.IONIC FORMULASYou can deduce the formula of an ioniccompound if you know the charges on theions involved. The total positive chargemust balance out the total negative chargeso you must look for the lowest commonmultiple (LCM) of the charges.Eg1. Calcium nitrate is made of Ca2 ions andNO3- ions. The LCM of 2 and 1 is 2 whichmeans you need 1 Ca2 ion and 2 NO3- ionsso the formula is Ca(NO3)2Eg2. Aluminium oxide is made of Al3 ionsand O2- ions. The LCM of 2 and 3 is 6 whichmeans you need 2 Al3 ions and 3 O2- ions sothe formula is Al2O3.Example 1: Water, H2OThe Ar for H and O are 1.01 and 16.00 so:Mr(H2O) 2 x 1.01 1 x 16.00 18.02Example 2: Magnesium Hydroxide, Mg(OH)2The Ar for Mg, O and H are 24.31, 16.00 and 1.01:Mr(Mg(OH)2) 1 x 24.31 2 x 16.00 2 x 1.01 58.33The relative formula mass (Mr) is the combined Ar Example 3: Decane, CH3(CH2)8CH3of all the elements in the formula for a substance. The Ar for C and H are 12.01 and 1.01Mr also has no units for the same reason as above. Mr(decane) 10 x 12.01 22 x 1.01 142.34
C4: STOICHIOMETRY – TheMole ConceptTHE MOLEA mole is 6.02x1023 of something. It is chosen so that a mole of something has the same mass ingrams (molar mass, Mm) as its formula mass. For example the Mr of water is 18.02 so the Mm of wateris 18.02g; the Mr of decane is 142.34 so the Mm of decane is 142.34g. Importantly this means that18.02 g of water and 142.34g decane contains the same number of molecules.EQUATIONS AND MOLE RATIOSEquations can be used to help us calculate the numbers of moles of substancesinvolved in a reaction. We can see this by studying the following reaction:2C2H6 7O2 4CO2 6H2OQ1: How many moles of CO2 are produced by burning 1.0 mol of C2H6? We say thatC2H6 is our ‘known’ and CO2 is our ‘unknown’ so:Moles CO2 moles known/knowns in eqn x unknowns in eqn 1.0 /2 x 4 1.0 x 2 2.0 molQ2: If 0.01 mol of CO2 is produced, how much H2O must also be produced? Thistime CO2 is our known and H2O is our unknown so:Moles H2O moles known/knowns in eqn x unknowns in eqn 0.01/4 x 6 0.0025 x 6 0.015 mol*You must make sure your equation is balanced or your mole ratio will be wrong.CALCULATING REACTING QUANTITIESUsing what we know about calculating moles, we can now answer questions like: IfI have 100g X, how much Y is made? The key is to convert the known to moles 1st.Example: What volume of H2 gas would be produced by reacting 12.15gmagnesium with excess hydrochloric acid? First we need a balanced equation:Mg 2HCl MgCl2 H2Then calculate moles of Mg (our known) we start with:Moles Mg mass/molar mass 12.15/24.30 0.50 molNext we work out how many moles of H2 ( our unknown) we expect to produce:Moles H2 moles known/knowns in eqn x unknowns in eqn 0.50/1 x 1 0.50 molFinally we calculate the volume using our equations for a gas:Volume H2 moles x 24.0 0.50 x 24.0 12.0 dm3LIMITING REACTANTSmoles of H2O could you make from 3 molThis is the reactant that will run out first. of H2 and 3 mol of O2. H2: 3/2 1.5, O2:It is important as this
CHEMISTRY REVISION GUIDE for CIE IGCSE Coordinated Science (2012 Syllabus) Whilst this guide is intended to help with your revision, it should not be your only revision. It is intended as a starting point but onlyastartingpoint. You should make sure that you also read your text books and use the internet to supplement your study in conjunction with
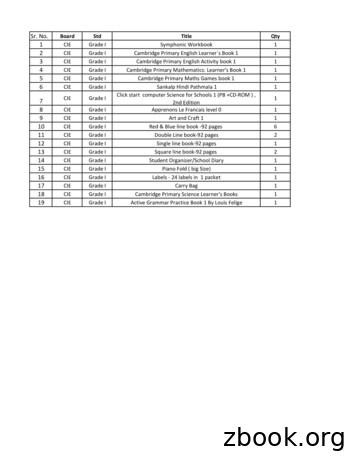
Sr. No. Board Std Title Qty 1 CIE Grade II Cambridge Primary English Learners Book 2 1 2 CIE Grade II Cambridge Primary English Activity book 2 1 3 CIE Grade II Cambridge Primary Mathematics: Learner's Book 2 1 4 CIE Grade II Cambridge Primary Maths Games book 2 1 5 CIE Grade II Sankalp Hindi Pathmala 2 1 6 CIE Grade II Click start computer Sc
Bruksanvisning för bilstereo . Bruksanvisning for bilstereo . Instrukcja obsługi samochodowego odtwarzacza stereo . Operating Instructions for Car Stereo . 610-104 . SV . Bruksanvisning i original
Physical chemistry: Equilibria Physical chemistry: Reaction kinetics Inorganic chemistry: The Periodic Table: chemical periodicity Inorganic chemistry: Group 2 Inorganic chemistry: Group 17 Inorganic chemistry: An introduction to the chemistry of transition elements Inorganic chemistry: Nitrogen and sulfur Organic chemistry: Introductory topics
Chemistry ORU CH 210 Organic Chemistry I CHE 211 1,3 Chemistry OSU-OKC CH 210 Organic Chemistry I CHEM 2055 1,3,5 Chemistry OU CH 210 Organic Chemistry I CHEM 3064 1 Chemistry RCC CH 210 Organic Chemistry I CHEM 2115 1,3,5 Chemistry RSC CH 210 Organic Chemistry I CHEM 2103 1,3 Chemistry RSC CH 210 Organic Chemistry I CHEM 2112 1,3
10 tips och tricks för att lyckas med ert sap-projekt 20 SAPSANYTT 2/2015 De flesta projektledare känner säkert till Cobb’s paradox. Martin Cobb verkade som CIO för sekretariatet för Treasury Board of Canada 1995 då han ställde frågan
service i Norge och Finland drivs inom ramen för ett enskilt företag (NRK. 1 och Yleisradio), fin ns det i Sverige tre: Ett för tv (Sveriges Television , SVT ), ett för radio (Sveriges Radio , SR ) och ett för utbildnings program (Sveriges Utbildningsradio, UR, vilket till följd av sin begränsade storlek inte återfinns bland de 25 största
Hotell För hotell anges de tre klasserna A/B, C och D. Det betyder att den "normala" standarden C är acceptabel men att motiven för en högre standard är starka. Ljudklass C motsvarar de tidigare normkraven för hotell, ljudklass A/B motsvarar kraven för moderna hotell med hög standard och ljudklass D kan användas vid
evaluation of English Pronunciation and Phonetics for Communication (second edition) and English Phonology (second . textbook is English Phonology written and edited by Wang Wenzhen, which was first published by Shanghai Foreign Language Educational Press in 1999. It was modified and republished in 2008 and also came with a CD. 4 Polyglossia Volume 25, October 2013 2.4 Procedure and Data .