Iso 15189 Accredited Laboratories Fulfill The Jci Hospital-PDF Free Download
ISO 15189 International Standards. 2 "This International Standard, based upon ISO/IEC 17025 and ISO 9001, specifies requirements for quality and competence that are particular to medical laboratories. (ISO 15189:2012(E) Introduction Page) 15189:2012. Medical laboratories Requirements -
ISO/TC 212 15189 ISO/CASCO 17025 ISO Family of Quality Management Standards 13 ISO/TC207/SC1 14000 ISO/TC 210 13485 Environment Medical Devices Medical Laboratory Reference Laboratory ISO/TC 176 9000 ISO 15189 The Core of the 15189 are 15 Management Requirements and 8 Technical Requirements
ISO 15189:2012 WORKING DOCUMENT NOTES: 1. This working document is intended as a checklist for the assessor when conducting Medical Testing Laboratory Accreditation Assessments according to ISO 15189:2012. This standard incorporates all elements of ISO 15189:2012, ISO 22870:2016, ISO 9001:2008, and ISO/IEC 17025:2005 relevant to medical testing .File Size: 606KB
Con este objeto se publicó la Norma ISO 15189; se trata de una norma internacional, basada en las normas ISO/IEC 17025 e ISO 9001 y destinada al uso por los laboratorios clínicos en el desarrollo de sus sistemas de gestión de la calidad y la evaluación de sus propias competencias. En concreto, la Norma 15189:2013 Laboratorios Clínicos.File Size: 562KB
transaction of business’ in Subclause 3.14 of ISO 15489‑1:2016 (3,p.2). Figure S1. Distribution of conformance requirements in Subclause 4.15.2 (Review input) of ISO 15189:2012 over the strategic management stage of the ISO 15189
ISO 15189: 2012 -MANAGEMENT REVIEW MEETING -I Meeting -U" Audit Team Members and Audited labs/Support services: Internal audit conducted based on new standard ISO 15189 :2012 forthefollowing departments Quality System: :MS.K.Vanitha Front Office & Preanalytical area :MS.R.Punitham Clinical Pathology andHematology :MS.R.Punitham .
ISO 15189 is based on ISO/IEC 17025 (General requirements for the competence of testing and calibration laboratories) and ISO 9001 (Quality management systems – Requirements). It therefore incorporates the quality systems elements addressed in ISO 9001 certification, as
ISO 10381-1:2002 da ISO 10381-2:2002 da ISO 10381-3:2001 da ISO 10381-4:2003 da ISO 10381-5:2001 da ISO 10381-6:1993 da ISO 10381-7:2005 ne ISO 10381-8:2006 ne ISO/DIS 18512:2006 ne ISO 5667-13 da ISO 5667-15 da Priprema uzoraka za laboratorijske analize u skladu s normama: HRN ISO 11464:2004 ne ISO 14507:2003 ne ISO/DIS 16720:2005 ne
ISO 10771-1 ISO 16860 ISO 16889 ISO 18413 ISO 23181 ISO 2941 ISO 2942 ISO 2943 ISO 3724 ISO 3968 ISO 4405 ISO 4406 ISO 4407 ISO 16232-7 DIN 51777 PASSION TO PERFORM PASSION TO PERFORM www.mp ltri.com HEADQUARTERS MP Filtri S.p.A. Via 1 Maggio, 3 20060 Pessano con Bornago (MI) Italy 39 02 957
Standard ISO 15189:2012 Medical laboratories - Requirements for quality and competence. This accreditation demonstrates technical competence for a defined scope and the operation of a laboratory quality management system (refer to joint ISO-ILAC-IAF Communiqué dated Ja
Development of ISO 15189 Written by medical laboratory professionals (ISO TC 212 : WG1) It has its origins in two ISO standards ISO 9001 and ISO 17025 Requirements for quality and competence of medical labs It’
While the standard is based on ISO/IEC 17025 and ISO 9001, it is a unique document that takes into consideration the specific requirements of the medical environment and the importance of the medical laboratory to patient care. Providing ISO 15189 Consulting And Tra
Page 2 of 17 EN ISO 15189:2012 standard in one hand and EN ISO 9001 and EN ISO 13485 standards on the other hand EN ISO 15189:2012 standard on “Medical Laboratories - Requirements for quality and competence” shares a number of similarities wi
ISO 18400-107, ISO 18400-202, ISO 18400-203 and ISO 18400-206, cancels and replaces the first editions of ISO 10381-1:2002, ISO 10381-4:2003, ISO 10381-5:2005, ISO 10381-6:2009 and ISO 10381-8:2006, which have been structurally and technically revised. The new ISO 18400 series is based on a modular structure and cannot be compared to the ISO 10381
The DIN Standards corresponding to the International Standards referred to in clause 2 and in the bibliog-raphy of the EN are as follows: ISO Standard DIN Standard ISO 225 DIN EN 20225 ISO 724 DIN ISO 724 ISO 898-1 DIN EN ISO 898-1 ISO 3269 DIN EN ISO 3269 ISO 3506-1 DIN EN ISO 3506-1 ISO 4042 DIN
ISO 15189 Medical laboratories – Particular requirements for quality and competence is a standard that contains the requirements necessary for diagnostic medical laboratories to demonstrate their competence to deliver reliable services. The scope of ISO 15189 states the standard is for
ISO 15189 2012 standard accreditation of the laboratory is introduced to create confidence among the patient, institution based customer, clinician, and other users. When a laboratory is accredited, it is considered that it has implemented all the requirements of the quality
ISO 15189:2012(E) Introduction This International Standard, based upon ISO/IEC 17025 and ISO 9001, specifies requirements for competence and quality that are particular to medical laboratori
CAP 15189 eceber, 2015 2 Background—Why This is Necessary The ISO 15189:2012 standard contains enhanced expectations regarding measurement uncertainty (MU) in clause 5.5.1.4. To clarify the laboratory’s responsibility and the CAP’s 15189 assessment standards, we have developed this
ISO 8402 was published in 1986, with ISO 9000, ISO 9001, ISO 9002, ISO 9003 and ISO 9004 being published in 1987. Further feedback indicated that there was a need to provide users with application guidance for implementing ISO 9001, ISO 9002 and ISO 9003. It was then agreed to re-number ISO 9000 as ISO 9000-1, and to develop ISO 9000-2 as the .
The Blood Bank in Our Lady’s Hospital, Navan has been accredited to ISO 15189 since 17th February 2009. The scope of the accreditation is detailed in Registration Number 215MT. All work relevant to the scope of ISO 15189 (current version
ISO 37120. PAS 181/ISO 37106. PAS 183 – data sharing & IT. PAS 184. PAS 185. a security-minded approach. ISO/IEC 30145 . reference architecture. ISO/IEC . 30146. ISO 37151. ISO 37153. ISO 37156. Data exchange. ISO 37154. ISO 37157. ISO 37158. Monitor and analyse . data. PAS 182/ ISO/IEC 30182. PD 8101. PAS 212. Hypercat. BIM. PAS 184. Role of .
ISO 14644‐1 FEDERAL STANDARD 209E ISO Class English Metric ISO 1 ISO 2 ISO 31 M1.5 ISO 410 M2.5 ISO 5 100 M3.5 ISO 6 1,000 M4.5 ISO 7 10,000 M5.5 ISO 8 100,000 M6.5 ISO 9N/A N/A Standard 209E classifications are out‐of‐date. This standard was officially retired in 2001. Increasing Cleanliness
ISO 45001 Established:-ISO 10006 -Quality in project management-ISO 10007 -Configuration management-ISO 15161 -Food safety (ISO 9000 and HACCP)-ISO 19600 -Compliance management systems-ISO 20000 -IT services-ISO 20121 -Sustainable event management-ISO 20400 -Sustainable purchasing-ISO 22000 -Food safety-ISO 22301 -Business continuity management
PS10 Section ISO 17025 ISO 15189 ISO 17034 ISO 17020, ISO 17021-1, ISO 17024, ISO 17065 3 Documents to upload as a new applicant All new applicants Please submit in advance of visit through INAB portal 4 Documents required for each team member on the day of the visit All CABs. Please submit in advance for initial assessment and reassessment (head office only; not required for witnessed .
the UK almost implemented ISO 15189 and ISO 22870 (27). However, countries have differences in quality achievement, assessment practices and implementation strategies (27). The accreditation bodies for performance requirements (9) grant verifications for meeting the min
ISO 14001, ISO 50001, ISO 26000, ISO 10002, ISO 16949 Kristina Zheliba Dicle Solmaz 05.10.20171
ISO 9001:2015 QMS and ISO 14001:2015 EMS and ISO 45001:2018 Internal audit 6. Principals of Quality Management System-ISO 9001:2015 7. ISO 9001 and 14001 and ISO 45001:2018 EQHSMS audit records 8. Table of Documented information Summary against ISO 9001:2015 and ISO 14001:2015 require
ISO/IEC Date: 2018-04-30 ISO/IEC_2018 TMB ISO/IEC Directives, Part 1 — Consolidated ISO Supplement — Procedures specific to ISO Directives ISO/IEC, Partie 1 — Supplément ISO consolidé — Procédures spécifiques à l’ISO Ninth edition, 2018 [Based on the fourteenth edition (2018
ISO 50003 (Auditing) ISO 50002 (Energy audits) ISO 50006 (Baseline and EnPIs) ISO 50015 (M&V Guidance) ISO 50007 (Energy Services) ISO 17747 (M&V Org) 10 Evaluation of energy savings documents Framework ISO 17743 Guidance Regions ISO 17742 Guidance Projects ISO 17741 Guidance M&V Organizations ISO
ISO/TC 8 Ships and marine technology, ISO/TC 146 Air quality, ISO/TC 147 Water quality, ISO/TC 190 Soil quality & SC 4 on biological characterization of soil ISO/TC 207 Environmental management, ISO/TC 268 Sustainable cities and communities, ISO/TC 322 Sustainable finance. BIODIVERSITY - ISO Q&A session - 2020-03 .
ISO/IEC 17024 is the ISO standard which outlines how personnel certification programs should be conducted in general. ISO 18436-1 is the ISO standard which outlines how personnel certification should be conducted specifically for personnel engaged in Condition Monitoring and Diagnostics of Machines. ISO 18436-2 is the ISO standard .
an ISO 15189 management system and allowed ample time for laboratory employees to 1) become familiar with the system and 2) develop a sufficient evidentiary trail of documents that can be assessed. In a medical laboratory, depending on the
Labmate.net ERP LIMS Implementation of ISO-15189 Recommendations for Page 7 Protection of laboratory information systems (LIS) B.4.3 If data in other computer can be accessed through the LIS (e.g. pharmacy or medical records), there should be appropriate computer security
Department: Laboratory-Medical ISO 15189 Testing Accreditation No. 9900 Appendix to certificate No. 9900-05 Date of issue 09.03.2014 Version No. 2 Page No. 1 of: 17 The certificate attached is an integral part of the schedule and is numbered identically חפסנה רפסמל הה
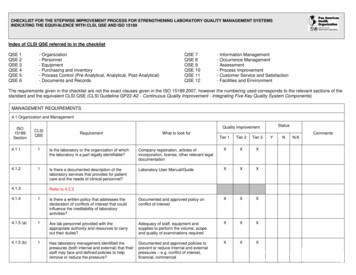
INDICATING THE EQUIVALENCE WITH CLSI, QSE AND ISO 15189 ISO 15189 Section CLSI QSE Requirement What to look for Quality Improvement Status Comments Tier 1 Tier 2 Tier 3 Y N N/A 4.2.4 1 Does the quality manual describe the quality management system and structure of the documentat
(Conjunto IAF-ILAC-ISO comunicado emitido en 2009). La correlación entre las cláusulas y subcláusulas de esta tercera edición de la OHN-ISO 15189 y los de la OHN-ISO 9001:2008 e OHN-ISO
What is MS ISO 15189:2012, Medical laboratories - Requirements for quality and competence? Specifies requirements for competence and quality that are particular to medical laboratories A medical laboratory’s fulfillment of
ISO 6974-1:2012, Annex A, provides a comparison of the characteristics of the analytical methods described in ISO 6974 -3 and subsequent parts of ISO 6974. It is intended that this part of ISO 6974 be used in conjunction with ISO 6974-1 and a method of analysis, e.g. ISO 6974-3 or subsequent parts of ISO 6974.
ISO 10381-1,2:2002, ISO 10381-3:2001, ISO 10381-4:2003, ISO 10381-5:2005, ISO 10381-6:2009, ISO 18512:2007 i Uputstvo za uzorkovanje MOL-LAB UP-1-14 SRPS EN ISO 5667-1:2008, SRPS EN ISO 5667-3:2007, SRPS EN ISO 5667-6:1997 i Uputstvo za uzorkovanje MOL-LAB UP-1-09 Datum prijema uzoraka: 23-24.08.2016. godine Opis, vrsta, broj