Academic Support Center CHEM 1020 Final Review Packet
Academic Support CenterCHEM 1020 Final Review PacketPage 1 of 8Naming Compounds1. Provide the name (problems a-h) or formula (problems i-q) for the following compounds.(a) Ca(NO3)2(e) K2SO4(j) silver phosphate(n) nickel(III) hydroxide(b) PCl5(f) Cu(ClO3)2(k) copper(II) bromide(o) cobalt(II) nitrate(c) Pb(CO3)2(g) Ba(C2H3O2)2(l) sodium sulfite(p) strontiumphosphide(d) S2Br2(h) AlF3(m) silicon dioxide2. Provide the name (problems a-c) or formula (problems d-f) for the following acids.(a) HBr(d) hydrofluoric acid(b) H3PO4(e) sulfurous acid(c) HClO2(f) nitric acid3. Acetone has a density of 0.7857 g/mL.(a) What is the mass in grams of 28.56 mL of acetone?(b) What is the volume in milliliters of 6.54 g of acetone?4. A paperback book has the dimensions 5.8 in x 2.0 in x 7.85 in. What is its volume in cubiccentimeters (cm3)?Chemical Equations5. Balance the following equations:C2H6 O2 CO2 H2OPCl5 H2O H3PO4 HClCHEM 1020 Final Review PacketUpdated Fall 2019 KA
Academic Support CenterCHEM 1020 Final Review PacketPage 2 of 86. A chemist adds a solution of hydrochloric acid to a solid sample of manganese(IV) oxide, andobserves the formation of chlorine gas, liquid water, and aqueous manganese(II) chloride. Write abalanced equation for this reaction with phase labels.Stoichiometry and Moles7. How many molecules of magnesium chloride are in a sample weighing 27.88 g? How many atoms ofjust chloride are in the sample?8. For the following reaction: 2 Cr2 O3 (𝑠) 3 C (𝑠) 4 Cr(𝑠) 3 CO2 (𝑔)(a) Find the moles of CO2 (𝑔) produced when we react 62.6 g of Cr2 O3 (𝑠).(b) How many grams of C would be needed to produce 25 g of Cr?9. Write the balanced reaction for the combustion of C4H10(g) with oxygen gas. Then, find how manygrams of water are produced when 43.8 g of C4H10(g) are reacted with 200. g of O2(g). What’s thelimiting reactant?10. A solution contains an unknown mass of dissolved barium ions. When sodium sulfate is added tothe solution, a white precipitate forms. The precipitate is filtered and dried and then found to have amass of 258 mg. What mass of barium was in the original solution? (Assume that all of the bariumwas precipitated out of solution by the reaction.)11. Draw the Lewis structure for the following elements:(a) lithium(b) sulfurCHEM 1020 Final Review PacketUpdated Fall 2019 KA(c) nitrogen
Academic Support CenterCHEM 1020 Final Review PacketPage 3 of 812. Use Lewis Theory to determine the formula for the compound that forms from:(a) Sr and S(b) Al and O13. For the following compounds, draw a Lewis dot structure, determine its electron geometry* andmolecular geometry*.FormulaLewis structureElectron Geometry*MolecularGeometry*CO2PCl3ClNO*Not covered in all 1020 sections14. Classify these molecules as polar or nonpolar. If you did not cover molecular geometry in yoursection, consult the key for the 3D shapes of these molecules.(a) CO2(b) PCl3(c) ClNOGas Laws15. Convert 46.38 kilopascales to barr.16. A balloon at 24 C has a volume of 14 L and a pressure of 785 mmHg. Assuming the balloon does notpop, what will its volume be on the on the summit of Mount Denali, where the temperature is 1.0 Cand the pressure is 350.5 mmHg?CHEM 1020 Final Review PacketUpdated Fall 2019 KA
Academic Support CenterCHEM 1020 Final Review PacketPage 4 of 8Organic Chemistry17. Draw the three isomers of hexyne.18. Classify each of the following as an alkane, an alkene, or an alkyne.(a) C4H8(b) C10H22(c) C3H419. Give the IUPAC name for each of the following:(a)(b) CH3CH2CH2-C CCH320. Classify each of the following as an alcohol, aldehyde, carboxylic acid, ester, ether, or ketone:(a) 3-pentanol(b) H3C-CH2-CH2-O-CH2-CH3(c) octanal(d) H3C-CH2-CH2-OH(e) 3-hexanoneO(f) H3C C O CH3O(g)CH3 CH2 CH2 C OHCHEM 1020 Final Review PacketUpdated Fall 2019 KA
Academic Support CenterCHEM 1020 Final Review PacketPage 5 of 821.For each of the following, determine whether the two structures are isomers or the samemolecule drawn in two different ways.CH3H3C(a)CHCHCH3CH3CH3H3C CH HCH2CH3H3CCH2 CHCH3CH3Liquids, Solids, and Intermolecular Forces22. What kind of intermolecular forces do each of the following exhibit?(a) HCl(b) HF(c) CCl423. Which has the highest boiling point: methane, ethane, or propane?Solutions24. Calculate the molarity of a solution that contains 67.9 g of NaCl in 2.00 L of solution25. Calculate the mass of NaCl in a 60.0 mL sample of 1.7 M NaCl.26. What volume of 12.0 M HCl is needed to prepare 250.0 mL of 0.500 M HCl?27. What is the concentration of a solution prepared by diluting 25.0 mL of 2.0 M NaOH to a volume of500.0 mL?CHEM 1020 Final Review PacketUpdated Fall 2019 KA
Academic Support CenterCHEM 1020 Final Review PacketPage 6 of 8Acids and Bases28. Identify the following as an acid or base by the Arrhenius definition(a) HNO3(b) KOH29. An aqueous solution has [𝑂𝐻 ] 3.3 10 5 M. What is the [𝐻3 𝑂 ] and pH of the solution?30. Calculate the pH of the following solutions:(a) 1.95 10 3 M HBr(b) 1.48 10 3 M KOH(c) 1.56 10 4 M Sr(OH)2Answers1.(a) calcium nitrate(b) phosphorouspentachloride(c) lead(IV) carbonate(d) disulfur dibromide(e) potassium sulfate(f) copper(II) chlorate(j) Ag3PO4(k) CuBr2(n) Ni(OH)3(o) Co(NO3)2(g) barium acetate(h) aluminum fluoride(l) Na2SO3(m) SiO2(p) Sr3P22.(a) hydrobromic acid(b) phosphoric acid(c) chlorous acid(d) HF(e) H2SO3(f) HNO33.(a) 22.44 g(b) 8.32 mL4.1500 cm35.2 C2H6 7 O2 4 CO2 6 H2OPCl5 4 H2O H3PO4 5 HCl6.4 HCl(aq) MnO2(s) Cl2(g) 2 H2O(l) MnCl2(aq)7.1.763 1023 molecules of MgCl2 and 3.527 1023 atoms of Cl-CHEM 1020 Final Review PacketUpdated Fall 2019 KA
Academic Support CenterCHEM 1020 Final Review PacketPage 7 of 88.(a) 6.18x10-1 mol CO29.Balanced equation: 2 C4H10 13 O2 8 CO2(g) 10 H2O (g); C4H10 is limiting, so 67.9 g of H2Oproduced.10.0.152 g Ba2 11.12.(a)(b) 4.3g C(b)(c)(a) SrS(b) Al2O313. Lewis structures for covalent compounds. Fill in the tableFormulaLewis structureElectron GeometryLinearCO2MolecularGeometryLinear (same aslewis dot structure)TetrahedralTrigonal pyramidalTrigonal planarBentPCl3ClNO14.(a) nonpolar15.0.4638 bar(b) polar(c) polarCHEM 1020 Final Review PacketUpdated Fall 2019 KA
Academic Support Center16.CHEM 1020 Final Review PacketPage 8 of 829 LCH3CH317. HCH3CH3C18.(a) alkene(b) alkane19.(a) 3,5-dimethyl-4-propyloctane (b) 2-hexyne20.(a) alcohol(b) ether(c) aldehyde(e) ketone(f) ester(g) carboxylic acid21.(a) same(b) isomers(c) same22.(a) dispersion, dipole-dipoleCH3(c) alkyne(d) alcohol(b) dispersion, dipole-dipole, hydrogen bonding(c) dispersion23.propane (CH3CH2CH3)24.0.581 M25.6.0 g NaCl26.10.4 mL of 12.0 M HCl needed27.0.10 M NaOH28.(a) acidb. base29.3.0x10-10 MpH 9.5230.a. 2.710b. 11.170c. 10.494CHEM 1020 Final Review PacketUpdated Fall 2019 KA
12. Use Lewis Theory to determine the formula for the compound that forms from: (a) Sr and S (b) Al and O 13. For the following compounds, draw a Lewis dot structure, determine its electron geometry* and molecular geometry*. Formula Lewis structure Electron Geometry* Molecular Ge
CHEM 350B Topics in Chemistry 7.5 454.95 CHEM 351 Chemicals Big and Small: Nano- 15 909.90 CHEM 352 Advanced Concepts in Chemistry 15 909.90 CHEM 352A Advanced Concepts in Chemistry 7.5 454.95 CHEM 352B Advanced Concepts in Chemistry 7.5 454.95 CHEM 360 Contemporary Green Chemistry 15 909.90 CHEM 380 Materials Chemistry 15 909.90
CHEM 31X. Chemical Principles 4 CHEM 33. Structure and Reactivity 4 CHEM 35. Organic Monofunctional Compounds 4 CHEM 36. Organic Chemistry Laboratory I 3 MATH 41, 42, 51. Calculus, Linear Equations 5 5 5 SECOND YEAR CHEM 130. Organic Chemistry Laboratory II 4 CHEM 131. Organic Polyfunctional Compounds y3 CHEM 134.
of the boiler, has no effect on the detector. The IRD 1020.1 replaces the IRD 1020 and 920. When exchanging the IRD 1020, care must be taken that the corresponding IRD 1020.1 with the same imprint (blue or white) is used (see page 3 and 4). When exchanging the IRD 920, care must be taken to wire the sensor correctly (see page 3), and the .
CHEM 110 Chemistry of the Living World 15 4,736.85 CHEM 120 Chemistry of Material World 15 4,736.85 CHEM 150 Concepts in Chemistry 15 4,736.85 CHEM 200 Special Topic 15 4,736.85 CHEM 251 Structure and Spectroscopy 15 4,736.85 CHEM 252 Properties and Analysis of Mat 15 4,736.85
WRF-Chem Version 3.9.1.1 User’s Guide Table of Contents 1.1 WRF-Chem Introduction3 1.2 WRF-Chem software 5 1.3 Possible applications of the current modeling system 5 1.4 The WRF-Chem modeling system overview 5 2.1 Software Installation Introduction 8 2.2 Building the WRF-Chem code 9 2.2.1 Getting the code 9
bonding and reactions) necessary for courses in elementary organic chemistry and physiological chemistry. Students may only receive credit toward graduation for one of the following: CHEM 10050; or CHEM 10060 and CHEM 10061; or CHEM 10970 and CHEM 10971.
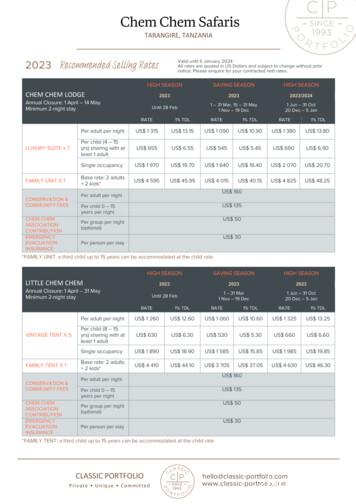
Chem Chem Safaris TARANGIRE, TANZANIA Updated: 9 November 2022 Page: 2 of 6 www.classic-portfolio.com CLASSIC PORTFOLIO Private Unique Committed hello@classic-portfolio.com www.classic-portfolio.com FOREST CHEM CHEM Annual Closure: 1 April - 31 May Minimum 2-night stay SAVING SEASON HIGH SEASON 2023 2023/2024 1 - 31 Mar 1 Nov - 19 Dec
The Institute of Food and Agricultural Sciences (IFAS) is an Equal Opportunity Institution authorized to provide research, educational information and . 1910.1020(f) -- Trade Secrets 1910.1020(g) -- Employee Information 1910.1020(h) -- Transfer of Records 1910.1020(i) -- Appendices . pertaining to employees exposed to toxic .