SN Kansagra School - Weebly
SN Kansagra SchoolCHEMISTRYCHAPTER - 2STD. XCHEMICAL BONDINGChemical bond is the force which holds two or more atoms or ions together in a stable molecule.AN ATOMAn atom is the smallest unit of matter taking part in a chemical reaction and is built up of subatomicparticles- protons, neutrons and electrons.Two or more atoms [metallic or non metallic] combine to form a molecule.The force which holds the atoms together as a stable molecule is a chemical bond.Types of elements- involved in chemical combination.METALS: have 1, 2 or 3 electrons in valence shell. They lose 1, 2 or 3 electrons and become positivelycharged ions (cations).NON METALS: have 4, 5, 6 or 7 electrons in valence shell. They gain 3, 2 or 1 electrons and becomenegatively charged ions (anions).Chemical combination: Atoms combine to form a molecule by two means:1.Transfer of valence electrons form a metallic atom to a non metallic atom.2.Sharing of valence electrons between two atoms (generally both non metallic).1
LEWIS THEORY OF CHEMICAL BONDINGIn 1916, Lewis put forth a comprehensive theory of bonding based on the electronic concept of theatom. According to him, bonding of an atom depends chiefly on the electrons in the outermost shell.He observed that the number of electrons in the last orbits of inert gases formed a most stablegrouping. Except the inert gases, all other elements have atoms with unstable and incomplete outershell. They tend to lose or gain electrons so as to acquire an electronic configuration identical withthat of the nearest inert gas in the periodic table having 8 electrons in the outermost orbit, excepthelium which contains 2 electrons. It is this tendency of atoms to complete and thus, stabilise theiroutermost ring of electrons which causes them to combine chemically. This theory has been appliedto explain the three common types of bonds.(1) Electrovalent bond(2) Covalent bond(3) Coordinate bondMETHODS FOR ACHIEVING CHEMICAL BONDINGA stable electronic configuration in two combining atoms resulting in chemical bonding betweenthem is achieved by:Electron transfer – of valence electrons from one atom to another leading to the formation ofelectrovalent bond and formation of electrovalent compound.Electron sharing – of pairs of electrons between two reacting atoms leading to covalent bonding andformation of covalent compound.2
PERIODIC PROPERTIES WHICH AFFECT THE FORMATION OF CHEMICAL BONDFor the formation of ionic bondIonisation potentialLower the value of I.P. of a metallic atom greaterthe ease of formation of the cation.Electron affinityHigher the value of E.A. of a non metallic atom,greater the ease of formation of the anion.ElectronegativityLarger the difference between two combiningatoms, electron transfer takes place easily.For the formation of Covalent bondIonisation potential, Electron affinity andElectronegativityElectronegativity differenceHigh between both the atoms.Should be negligible between two combiningatoms.TYPES OF CHEMICAL BONDS1. ELECTROVALENT BONDElectrovalent or ionic bond: Thechemical bond that is formed betweentwo atoms by transfer of one or moreelectrons from the atom of anelectropositive or metallic element tothe atom of an electronegative or nonmetallic element is called anelectrovalent or ionic bond.1.An electrovalent bond is formed by the complete transfer of electrons from one atom toanother.2.Transfer of electrons takes place from the atom of a metallic element to the atom of a nonmetallic element.3.Due to transfer of electrons, one atom loses electrons while other atom gains electrons,leading to the formation of charged particles called ions.4.There exists a strong force of attraction between oppositely charged particles, resulting inthe formation of an electrovalent bond.3
5.The compounds formed by transfer of electrons from one atom of metallic element toanother atom of a non metallic element is called an electrovalent or ionic compound.It is clear that for an ionic bond to form, there must exist an atom that readily loses one or moreelectrons, and another that readily accepts these electrons.Elements of group 1and 2 of the periodic table have the greatest tendency to lose electrons and getconverted into cations.Elements of group 16 and 17 of the periodic table have the greatest tendency to gain electrons andget converted into anions.PROPERTIES OF IONIC COMPOUNDS1.These compounds are obtained by the transfer of electrons from one atom to another andtherefore consists of charged particles.2.These compounds are generally soluble in water but insoluble in non-polar solvents likebenzene and ether.3.They possess high melting and boiling points.4.They conduct electricity in molten state and in solution.5.They are crystalline hard and brittle solids.6.The reactions of ionic compounds are in fact the reactions of their ions. These reactions takeplace at a rapid speed.4
FORMATION OF IONIC COMPOUNDS1. SODIUM CHLORIDESodium loses an electron to resemble the configuration of neon [2, 8] While chlorine takes up thatelectron to resemble the configuration of argon [2, 8, 8]. Sodium forms a cation and chlorine ananion. The oppositely charged ions are held together by strong electrostatic forces resulting in theformation of the electrovalent compound, Sodium chloride.Electron dot diagram:5
2. MAGNESIUM CHLORIDEMagnesium has two valence electrons which it must lose to resemble the configuration of the inertgas, neon. One chlorine atom requires one electron to form a stable octet. Hence, two chlorineatoms, each with a capacity to take up one electron are required to form the compound, magnesiumchloride.Electron dot diagram:6
3. CALCIUM OXIDECalcium needs to lose electrons from its outermost shell in order to resemble argon while oxygenneeds two electrons to attain the configuration of neon. Therefore, calcium atom loses twoelectrons which are taken up by oxygen and a stable, ionic compound, calcium oxide is formed.COVALENT BOND1.A covalent bond is formed by the mutual sharing of electron pairs between two atoms.2.The sharing of electron pairs take between atoms of non metallic elements. This is becauseboth of them are deficient in electrons and both have a tendency to gain electrons.Thus, transfer of electrons is not possible and the octet is satisfied by sharing of electrons.3.Atoms which in their outermost shells have seven, six and five electrons share one, two andthree pairs of electrons respectively forming covalent molecules.7
4.The sharing of electrons between atoms results in the formation of non ionised molecules.This is because electrons are not transferred from one atom to another, leading to theabsence of ions.5.The chemical compound, formed as a result of mutual sharing of electrons or electron pairsthereby establishing a covalent bond, is called a covalent or molecular compound.Covalency of an atom is the number of its electrons forming shared pairs with other atoms.Hydrogen moleculeOxygen moleculeCovalent bond: The chemical bond that is formed between two combining atoms by mutual sharingof one or more electrons of atoms of non-metallic elements is called a covalent or a molecular bond.FORMATION OF COVALENT MOLECULES:1. HYDROGEN:Each of two hydrogen atoms contributes one electron so as to have one shared pair of electronsbetween them. Both atoms attain stable duplet structure, resulting in the formation of a singlecovalent bond between them.Atomic or orbit structural diagram of hydrogen molecule formation8
Electron dot diagram of hydrogen molecule formation2. CHLORINE:Each of the two chlorine atoms contributes one electron so as to have one shared pair of electronsbetween them. Both atoms attain stable octet structure, resulting in the formation of a singlecovalent bond between them.Atomic or orbit structural diagram of Chlorine molecule formationElectron dot diagram of hydrogen molecule formation9
3. OXYGEN:Each of the two oxygen atoms contributes two electrons so as to have two shared pair of electronsbetween them. Both atoms attain stable octet structure, resulting in the formation of a doublecovalent bond between them.Atomic or orbit structural diagram of oxygen molecule formationElectron dot diagram of oxygen molecule formation10
4. NITROGEN:Each of the two nitrogen atoms contributes three electrons so as to have three shared pair ofelectrons between them. Both atoms attain stable octet structure, resulting in the formation of atriple covalent bond between them.Atomic or orbit structural diagram of Nitrogen molecule formationElectron dot diagram of Nitrogen molecule formation5. CARBON TETRACHLORIDE:Four atoms of chlorine and one atom of carbon combine to form one molecule of carbontetrachloride. Carbon has four valence electrons and hence requires four electrons to complete itsoctet. Chlorine has seven valence electrons and requires only one electron to complete its shell.Therefore, four chlorine atoms share one electron each with carbon. Thus, both, carbon andchlorine, complete their valence shells with eight electrons.11
6. METHANE:Four atoms of hydrogen and one atom of carbon combine to form one molecule of methane. Carbonhas four valence electrons and hence requires four electrons to complete its octet. Hydrogen hasone valence electron and requires only one electron to complete its shell. Therefore, four hydrogenatoms share one electron each with carbon. Thus, carbon completes its valence shells with eightelectrons and hydrogen completes its valence shell with two electrons.POLAR AND NON-POLAR COVALENT COMPOUNDSNon polar covalent compoundsCovalent compounds are said to be non polarwhen shared pair of electrons are equallydistributed between the two atoms.No charge separation takes place. The covalentmolecule is symmetrical and electricallyneutral.Polar covalent compoundsCovalent compounds are said to be polar whenshared pair of electrons are unequally distributedbetween the two atoms.Charge separation takes place. The atom whichattracts electrons more strongly develops a slightnegative charge.e.g. H2, Cl2, O2, N2, CH4, CCl4.e.g. H2O, NH3, HCl.12
Coordinate bonds (Dative bonds)Coordinate bond or Dative bond is formed by sharing of two electrons between two atoms whereboth the electrons of the shared pair are contributed by one atom and another atom merelyparticipates in sharing.This is a special type of covalent bond.The atom which contributes the shared pair of electrons is called the donor, while the other whichsimply shares the electron pair contributed by donor, is known as acceptor. The donor atom has acomplete octet in the valence shell but it still has one or more unshared pairs of valence electronscalled lone pairs of electrons. The donor contributes such a pair of electrons for being shared by theacceptor, which is short of two electrons in its valence shell. The bond formed in this way is generallyrepresented by an arrow () pointing from donor atom to the acceptor atom.FORMATION OF HYDRONIUM ION:A water molecule contains an oxygen atom having two lone pairs of electrons linked to twohydrogen atoms through covalent bonds.The hydrogen ion (H ) in hydronium ion shares one lone pair of electrons of the oxygen atom ofwater molecule. This results in the formation ofH3O ion.Lewis electron-dot representation:FORMATION OF AMMONIUM ION:An Ammonia molecule contains a nitrogen atom having one lone pair of electron linked to threehydrogen atoms through covalent bonds.The hydrogen ion (H ) in ammonium ion shares one lone pair of electrons of the nitrogen atom ofammonia molecule. This results in the formation ofNH4 ion.Lewis electron-dot representation:13
Two or more atoms [metallic or non metallic] combine to form a molecule. The force which holds the atoms together as a stable molecule is a chemical bond. Types of elements- involved in chemical combination. METALS: have 1, 2 or 3 electrons in valence shell. They lose 1, 2 or
2012-13, STD 11 CLASSIFICATION Page 1 Dichotomous Keys Using Smiley Faces Instructions: Use the key below t
COVID-19 Impact on Chronic Disease Susan Kansagra, MD, MBA, section chief, Chronic Disease and Injury, Division of Public Health, North Carolina Department of Health and Human Services Cardiovascular Disease Management Keith C. Ferdinand, MD, FACC, FAHA, FASPC, FNLA, professor of medicine, Tulane University School of Medicine
Weebly Objectives: Students will be able to: Learn about Weebly Create an eportfolio using weebly Upload their work and artifacts onto their eportfolio Add images, text, dividers, spacers, etc. 1. Go to Weebly.com and sign in with your google information. If it doesn’t work, sign up! 2. You can either Sign up
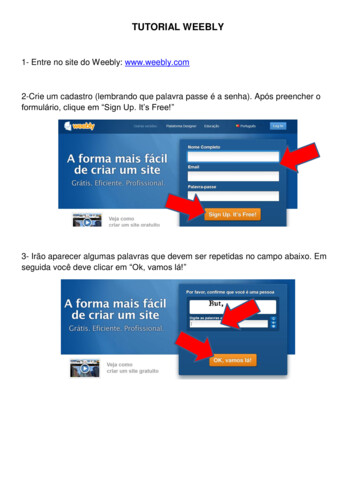
TUTORIAL WEEBLY 1- Entre no site do Weebly: www.weebly.com 2-Crie um cadastro (lembrando que palavra passe é a senha). Após preencher o formulário, clique em “Sign Up. It’s Free!” 3- Irão aparecer al
Dwight Elementary School Burr Elementary School King Street Intermediate School Western CT Academy of International Studies Magnet Broadview Middle School Rogers Park Middle School Pathways Academy Westside Middle School Academy Great Plain School Shelter Rock School (2011 & 2014) King Street Primary School Ellsworth Ave School Pembroke Stadley .
mead school district 354 mercer island school dist 400 meridian school district 505 monroe school district 103 morton school district 214 mossyrock school district 206 mt baker school district 507 mt vernon school district 320 mukilteo school district 6 napavine school district 14 newport school district 56-415 nooksack valley sch dist 506
Adams Lillian Nordman 1937 Nashua High School Adams Patricia 1957 Nashua High School Adams Richard 1957 Nashua High School Adams Shawndelle 2010 Nashua-Plainfield Adams Spencer 2008 Nashua-Plainfield Adelmund Jerry 1987 Nashua High School Adelmund Kay L. Clancy 1983 Nashua High School Adelmund Kimberly Shields 1979 Nashua High School
build-up and as a follow-up to the 11th World Trade Organization (WTO) Ministerial Conference (MC11) in December 2017. At MC11 in Buenos Aires, differences on digital commerce could not be bridged. Views were significantly opposed. Discussions were heated. While negotiators cannot reach compromise let alone consensus, the digital economy continues to grow very fast, with major economic and .