Artificial Photosynthesis: A Synthetic Organic Perspective
Artificial Photosynthesis: a synthetic organic perspectiveWei LiuMacMillan group meetingMar. 11th, 2020image credit: Wiley-VCH
Photosynthesis: a brief overviewNet reaction6 CO2 6 H2OOHHOsunlightHHH OHOHHOH 6 O2OHWater-splitting reaction (Light-dependent)sunlight2 H2OO2 4 H 4 e-(stored in NADPH and ATP)CO2 fixation (Light-independent)OHRuBisCO3 CO2-O PO3CHO(G3P)Jack Twilton, MacMillan group meeting, “Photosynthesis – An Organic Chemists Guide”
Artificial photosynthesis: a brief overviewOutlookArtificial photosynthesissunlight2 H2OO2 2 H2OHHOHchemicalCO2 H2COCH4CH3OHHH OHOHbiologicalC4H9OHHOHOHand more Complex organic moleculesSocioeconomic considerationsProgress in synthetic biologyRepresentative review: Chem. Rev. 2013, 113, 6621; Acc. Chem. Res. 2017, 50, 476
CO2 Conversion ChallengeStarted by NASA in 2019 (now in Phase II)Space exploration to MarsCO2 recycling (life support system)CO2 fixation (availabe SM on Mars)Why NASA wants glucose?Energy sources for microbial fermentationC6 sugar is preferred in microbial systemTotal synthesis of glucose on Mars?What about earthly synthesis of glucose?https://www.co2conversionchallenge.org
Nature’s approach: Calvin-Benson cycleRuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase)OOHPOOPOHRuBisCOOHPOCO2, H2Oresponsible for more than 90% carbon fixationone of the most abdundant enzyme on earthlow turnover frequency (1 to 10 s-1)3 to 10 molecules of CO2 fixed per second per enzymelow specificity (CO2 vs O2)targets for synthetic biologyErb, T. J.; Zarzycki, J. Curr. Opin. Biotech. 2018, 49, 100Bar-Even, A.; Noor, E.; Lewis, N. E.; Milo, R. Proc. Natl. Acad. Sci. USA 2010, 107, 8889Schwander T.; Schada, v. B. L.; Burgener, S.; Cortina, N. S.; Erb, T. J. Science, 2016, 354, 900OHO
Nature’s approach: Calvin-Benson cycleMechanism of RuBisCOOOHPOOHenolizationOPPOOHCO2OPMg2 OHOHOHPOOP–OOCOHORibulose-1,5-BPOHH 2 to C6 sugarOHHPOOcross AldolO s of RuBisCO3 Ribulose-1,5-BP 3 x C53 CO25 G3P1 G3P3 x C15 x C31 x C3Erb, T. J.; Zarzycki, J. Curr. Opin. Biotech. 2018, 49, 100
Nature’s approach: Calvin-Benson cycleMechanism of RuBisCOOOHPOOHenolizationOPPOOPMg2 OHOHOHO2POOHOOH Ribulose-1,5-BPrecycleOHPOOCO2 to C6 sugarOHOHHPOOO HOOHPOOHOHOHtautomerizationOFructose-6-PEconomics of RuBisCO3 Ribulose-1,5-BP 3 x C53 CO25 G3P1 G3P3 x C15 x C31 x C3Erb, T. J.; Zarzycki, J. Curr. Opin. Biotech. 2018, 49, 100
De novo synthesis of HexoseOHasymmetric epoxidationOHHOOHOHOOHOHHMasamune & Sharpless, Science 1983, 220, 949OOMeOOBn[4 2] cycloaddition OHOOBnR1OAcOAcORORBoger, J. Org. Chem. 1988, 53, TIPSOOHOAcOHMacMillan, Science 2004, 305, 1752
De novo synthesis of HexoseTi(OiPr)4, tBuOOHOHROOHROO( )-diisopropyl tartrateMeOOHPhSH, NaOHSPhROtBuOHOHOMeOMeMe1. mCPBASPhROPOCl3ORO2. Ac2O, NaOAcODIBALSPhOOAcO1.OROCHOPh3POHOOOHSPhROOOHOO1. Diol protection2. Pummerer3. K2CO3, MeOH4. deprotectionOHROOPhSH, NaOHtBuOHOHRO2. NaBH4O(-)-AEOHOHOHOOHOHKo, S. Y.; Lee, A. W. M.; Masamune, S.; Reed, L. A.; Sharpless, K. B. Science 1983, 220, 949L-glucose
De novo synthesis of HexoseTi(OiPr)4, tBuOOHOHROOHROO( )-diisopropyl tartrateROOHOH-OROOtBuOHSPhROOHOHPhS-SPhROOHPayne rearrangementOHPhSH, NaOHOHavoids unprotected triolKo, S. Y.; Lee, A. W. M.; Masamune, S.; Reed, L. A.; Sharpless, K. B. Science 1983, 220, 949
De novo synthesis of HexoseOHasymmetric epoxidationOHHOOHOHOOHOHHMasamune & Sharpless, Science 1983, 220, 949OOMeOOBn[4 2] cycloaddition OHOOBnR1OAcOAcORORBoger, J. Org. Chem. 1988, 53, TIPSOOHOAcOHMacMillan, Science 2004, 305, 1752
De novo synthesis of HexoseInverse electron demand [4 2] cycloadditionOOOH3COOEtOH3CO OCH3OCH3ConditionResultEtAlCl2 or TiCl4complex mixtureToluene, 110oC48% yield (2:1 endo:exo)neat, 13 kbar82% yield (6:1 endo:exo)CH2Cl2, 6 kbar75% yield (6:1 endo:exo)OOH3COOEtOOEt13 kbarOAcCH2Cl2H3COOOEt OAcOCH3OCH349% yield ( 45:1 endo:exo)Boger, D. L.; Robarge, K. D. J. Org. Chem. 1988, 53, 5793
De novo synthesis of HexoseH2MeO2COOEtLAHOHOOOEtOH3COOEtOCH3OCH313 OEtOCH3OAc13 kbarOCH3LAH;AcOOOEtOAcAc2OOCH3Boger, D. L.; Robarge, K. D. J. Org. Chem. 1988, 53, 5793BH3[O]OHOHOOEtOAcOCH3
De novo synthesis of HexoseOHasymmetric epoxidationOHHOOHOHOOHOHHMasamune & Sharpless, Science 1983, 220, 949OOMeOOBn[4 2] cycloaddition OHOOBnR1OAcOAcORORBoger, J. Org. Chem. 1988, 53, TIPSOOHOAcOHMacMillan, Science 2004, 305, 1752
De novo synthesis of ROROHOROR92% yield, 4:1 anti:syn, 95% ee (R TIPS)78% yield, 4:1 anti:syn, 98% ee (R Bn)MgBr2Et2OTIPSOOOH79% yieldTIPSOOAcOHOOHOTMS 10:1 dr95% eeL-mannose87% yieldOAcOHTiCl4L-glucoseOOH 19:1 dr95% eeL-allose97% yieldTIPSOOAcOH 19:1 dr95% eeNorthrup, A. B.; MacMillan, D. W. C. Science 2004, 305, 1752Northrup, A. B.; Mangion, I. K.; Hettche, F.; MacMillan, D. W. C. Angew. Chem. Int. Ed. 2004, 43, 2152
NASA’s rs.nasa.gov/19850008164.pdf
NASA’s approachpage a.gov/19850008164.pdf
Formose reactionaq. d by Aleksandr Butlerow in 1861Considered by NASA as a way to synthesize glycerolNow accepted mechanism proposed by Breslow in 1959Autocatalytic: long induction period, followed by fast reactionAddition of intermediates removes induction, but doesn’t increase rateOHOHHHOOHO OHOHa mixture of sugars: “formose sugar”OHOH2CHOOHOButlerow, A. Justus Liebigs Annalen der Chemie, 1861, 120, 295Breslow, R. Tetrahedron Lett. 1959, 21, 22OHOH
Formose reactionAutocatalytic cycleOOO OH)2OHHOHOOHOOHOAldolOCa(OH)2HHBreslow, R. Tetrahedron Lett. 1959, 21, 22
Formose reactionProduct-forming pathwayOHOHOOHHOHOHOOHOHOHHOOHOHCross Aldol o H2CHOOOHOHHOHBreslow, R. Tetrahedron Lett. 1959, 21, 22OHOH
Formose reactionThe realityOaq. OOHOHOHHOOOHOHHOOHHOOHOHOOHHHOOHHOOHand all the diastereomers ODecker, P.; Schweer, H.; Pohlmann, R. J. Chromatogr. A 1982, 244, 281
Formose reactionThe reality“the formose product can be regarded as a carbohydrate analog of petroleum, in that it containsso many carbohydrates of varying molecular weight and isomeric structure” (Weiss et al., 1970)Decker, P.; Schweer, H.; Pohlmann, R. J. Chromatogr. A 1982, 244, 281
Selective Formose reactionOOHOHHOHOHborate mineralsOHOHglycoaldehydeHHBC5 pentosestabilized by borate onor)HOOHNa2B4O7OHOC4 sugarstabilized by silicate complexOHOEtO3XHOthiazolium catalystHformaldehyde17% IYHOOHBr–NSdihydroxyacetoneRicardo, A.; Carrigan, M. A.; Olcott, A. N. Benner, S. A. Science 2004, 303, 196Lambert, J. B.; Gurusamy-Thangavelu, S. A.; Ma, K. Science 2010, 327, 984Matsumoto, T.; Yamamoto, H.; Inoue, S. J. Am. Chem. Soc. 1984, 106, 4829catalystSiOO
Selective Formose reactionOOHOOHCa(OH)2HHHOOHOHHO45oCOHOHOriginal Formose conditionOpentose detected after 20 minspentose disappeared after 1 hourOHHOOOHreaction turned brownHOBOOBorate mineral conditionpentose is the majority of total carbonribose remains stable for daysOHHHOdecomp.OHsuggest prebiotic ribose synthesisenediolRicardo, A.; Carrigan, M. A.; Olcott, A. N. Benner, S. A. Science 2004, 303, 196
Selective Formose reactionOHOOHOHNa2SiO3OHOHOH25oCHOHHmajor C4 with some C6 product after 20 minsHC6 becomes major product after 12 hoursOHHOOHNa2SiO3HHOOHOHOproduct decomposes or oligomerizes if it can’t form silicateOHO25oCOSiHOOOC6 sugar silicateLambert, J. B.; Gurusamy-Thangavelu, S. A.; Ma, K. Science 2010, 327, 984
Selective Formose reactionOHOOHHONa2SiO3HOHOHOH25oCOHsilicate, 30 minssilicate, 12 hoursNaOH, 30 minsNaOH, 12 hoursLambert, J. B.; Gurusamy-Thangavelu, S. A.; Ma, K. Science 2010, 327, 984
Selective Formose reactionEtthiazolium (5 %)O3XHOHOEt3N (5%), EtOH30 mins, EtHHOSOEtNHOSHCH2 NHO CSHHCHEtONCHNBr–SEtNHOHOOEtCH2 NCSEtHOHSONHOHOCSHOMatsumoto, T.; Yamamoto, H.; Inoue, S. J. Am. Chem. Soc. 1984, 106, 4829OH
Selective Formose reactionEtthiazolium (5 %)O3XHOHOEt3N (5%), EtOH30 mins, EtHHOSOEtNHOSHCH2 NHO CSHHCHEtONCHNBr–SEtNHOHOOEtCH2 NCSEtHOHSONHOHOCSHOMatsumoto, T.; Yamamoto, H.; Inoue, S. J. Am. Chem. Soc. 1984, 106, 4829OH
Selective Formose reactionthiazolium (5 %)O3XHOHOEt3N (5%), EtOH30 mins, 100oCHOformaldehydeOHHOOHHnot detecteddihydroxyacetoneReactivity with thiazolium:OOOH HOH HHHOCHOControl experiments:thiazolium (5 %)Ono conversionHOHHOEt3N (5%), EtOHthiazolium (5 %)OHno conversionCHOHOEt3N (5%), EtOHMatsumoto, T.; Yamamoto, H.; Inoue, S. J. Am. Chem. Soc. 1984, 106, 4829CHO
Selective Formose reactionthiazolium (5 %)O3XHOHOEt3N (5%), EtOH30 mins, 100oCHOformaldehydeOHHOOHHHOnot detecteddihydroxyacetoneReactivity with thiazolium:OOOH HOH HHHOCHOControl experiments: HHHOHOHHH HOOHOHOCHOOHEt3N (5%), EtOHthiazolium (5 %)OHOOthiazolium (5 %)OOOH HOEt3N (5%), EtOHCHOfully recoveredMatsumoto, T.; Yamamoto, H.; Inoue, S. J. Am. Chem. Soc. 1984, 106, 4829CHO
Selective Formose reactionthiazolium (5 %)O3XHOHOEt3N (5%), EtOH30 mins, 100oCHOformaldehydeOHHOOHHOHCHOnot detecteddihydroxyacetoneReactivity with thiazolium:OOOH HO HHHHOCHOControl experiments: HOthiazolium (5 %)OOHOHOHHOHEt3N (5%), EtOHEtIf formaldehyde reacts o, T.; Yamamoto, H.; Inoue, S. J. Am. Chem. Soc. 1984, 106, 4829OHHOCHOnot detected
MacMillan, Science 2004, 305, 1752. De novo synthesis of Hexose Boger, D. L.; Robarge, K. D. J. Org. Chem. 1988, 53, 5793 Inverse electron demand [4 2] cycloaddition H 3CO O O OCH 3 OEt O OEt OCH 3 H 3CO O Condition Result neat, 13 kbar 82% yield (6:1 end
Arti cial Neural Networks Human and Arti cial Neurons Arti cial Neurons ANN Artchitecture A simple neuron Arti cial neuron is a device with many inputs and one output Two modes: Training Using Firing Ruledetermines when a neuron should re. Are very important in neural networks and accounts for their high exibility
CHAPTER 1. INTRODUCTION Arti cial neural networks (https://www.merriam-webster.com) de nes as arti cial intelligence: 1. A branch of computer science dealing with the simulation of intelligent behavior in computers;
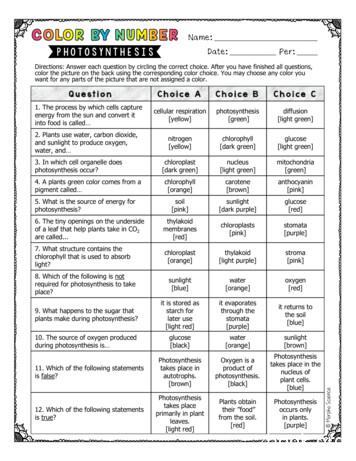
Photosynthesis takes place in autotrophs. [brown] Oxygen is a product of photosynthesis. [black] Photosynthesis takes place in the nucleus of plantcells. [blue] 12. Which of the followingstatements is true? Photosynthesis takes place primarily in plant leaves. [light red] Plants obtain their ”food” from the soil. [red] Photosynthesis .
photosynthesis 1/2-5/2 8/2 S1-5 Picnic Day 9/2-19/2 Lunar New Year Holiday photosynthesis 11A 4/6 Chapter 21 Photosynthesis o Basic concepts of photosynthesis o Requirements for photosynthesis o Site of The process of photosynthesis oPre Lesson Worksheet o Communication s
Photosynthesis Chapter 8 2 Photosynthesis Overview Energy for all life on Earth ultimately comes from photosynthesis. 6CO 2 12H 2O C 6H 12O 6 6H 2O 6O 2 Oxygenic photosynthesis is carried out by: cyanobacteria, 7 groups of algae, all land plants 3 Photosynthesis Overview Photosynt
UNIT 1 PHOTOSYNTHESIS AND RESPIRATION Photosynthesis is the process whereby plants use carbon dioxide, water and light energy in a series of chemical reactions to produce glucose (food). Photosynthesis Microorganisms Interactions and interdependencies Life and living Photosynthesis and respiration Photosynthesis Respiration
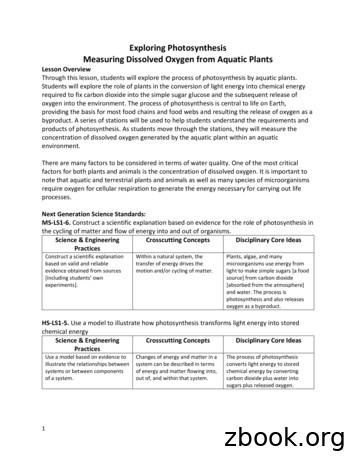
photosynthesis of an aquatic plant [Elodea]. The rate of photosynthesis can be determined by measuring the concentration of dissolved oxygen as the plant undergoes photosynthesis. There are multiple methods for measuring the rate of photosynthesis including: The uptake of CO 2 The production and release of O 2
In this live Gr 11 Life Sciences show we take a look at Photosynthesis. In this lesson we study the process of photosynthesis looking at the light and dark phases. We discuss the importance of photosynthesis as well as consider the effects of varying amou nts of light, carbon dioxide and temperature on the rate of photosynthesis.