Trends In Analytical Chemistry Vol 28 No 8 2009-PDF Free Download
Menschen Pagina 20 Schritte international Neu Pagina 22 Motive Pagina 24 Akademie Deutsch Pagina 25 Starten wir! Pagina 26 Themen aktuell Pagina 28 em neu Pagina 29 Sicher! Pagina 30 Vol A1 1 Vol A1 Vol 1 Vol 1 2 Vol unico Vol 1 Volume 1 Volume 1 Vol 1 Vol 1 1 Vol A1 2 Vol 2 Vol 1 2 Vol A2 1 Vol A2 Vol 3 Vol
Stage / Analytical Chemistry Lecture - 1 Introduction to Analytical Chemistry 1.1 Types of analytical chemistry & their uses . 1.2 Classifying Analytical Techniques. 1.3 Quantitative Analysis Methods. 1.4 Applications of Analytical Chemistry. 1.5 Units For Expressing Concentration of Solutions. 1.6 P Functions. 1.7 Stoichiometric Calculation.
Akenson, Donald Harman Vol 8: 10 Alan, Radous, at Agincourt Vol 12: 1 Albert, King Vol 7: 45, 47 Albert, Prince Vol 12: 17; Vol 14: 1 Alden, John Vol 5: 34; Vol 9: 18 Alexander III Vol 13: 24 Aleyn, John, at Agincourt Vol 12: 1 Allen, Pat Vol 10: 44 Alling Vol 4: 26 Amore, Shirley Vol 12: 3 Anderson, Robert Vol 10: 46 Anderson, Virginia DeJohn .
Chemistry ORU CH 210 Organic Chemistry I CHE 211 1,3 Chemistry OSU-OKC CH 210 Organic Chemistry I CHEM 2055 1,3,5 Chemistry OU CH 210 Organic Chemistry I CHEM 3064 1 Chemistry RCC CH 210 Organic Chemistry I CHEM 2115 1,3,5 Chemistry RSC CH 210 Organic Chemistry I CHEM 2103 1,3 Chemistry RSC CH 210 Organic Chemistry I CHEM 2112 1,3
Everything is made of chemicals. Analytical chemistry determine what and how much. In other words analytical chemistry is concerned with the separation, identification, and determination of the relative amounts of the components making up a sample. Analytical chemistry is concerned with the chemical characterization of matter and the
ADVANCED DIPLOMA Diploma in Chemistry 60% in Analytical Chemistry 3 Theory & Practical, Chemical Quality Assurance, Mathematics 2 Chemical Industrial 1 or S5 Subjects and Chemistry project II. Semester 1 Analytical Chemistry IV Physical Chemistry IV Research Methodology in Chemistry Semester 2 Inorganic Chemistry IV Organic Chemistry IV .
Physical chemistry: Equilibria Physical chemistry: Reaction kinetics Inorganic chemistry: The Periodic Table: chemical periodicity Inorganic chemistry: Group 2 Inorganic chemistry: Group 17 Inorganic chemistry: An introduction to the chemistry of transition elements Inorganic chemistry: Nitrogen and sulfur Organic chemistry: Introductory topics
of recent advances in green analytical chemistry as well as touch on some traditional methodologies that have always been environmentally benign, but perhaps not called green. 2. Trends in Green Analytical Chemistry Analytical chemistry provides the data necessary to make decisions about human and environmental health. Fast,
Fundamentals of Analytical Chemistry (9thEd, 2014) Skoog-West-Holler-Crouch 7 8 9. 4 Chapter 1 Introduction of Analytical Chemistry What is Analytical Chemistry? An unknown sample solution The sample could contain: (1) Ca2 , Na , or K ions OR (2) A urine sample from a potentially pregnant woman
Chemistry is the science that describes matter, its properties, the changes it undergoes, and the energy changes that accompany those processes. Inorganic chemistry Organic chemistry Physical chemistry Biochemistry Applied Chemistry: Analytical chemistry, Pharmaceutical Chemistry, . Istv an Szalai (E otv os University) Lecture 1 6 / 45
Accelerated Chemistry I and Accelerated Chemistry Lab I and Accelerated Chemistry II and Accelerated Chemistry Lab II (preferred sequence) CHEM 102 & CHEM 103 & CHEM 104 & CHEM 105 General Chemistry I and General Chemistry Lab I and General Chemistry II and General Chemistry Lab II (with advisor approval) Organic chemistry, select from: 9-10
CHEM 0350 Organic Chemistry 1 CHEM 0360 Organic Chemistry 1 CHEM 0500 Inorganic Chemistry 1 CHEM 1140 Physical Chemistry: Quantum Chemistry 1 1 . Chemistry at Brown equivalent or greater in scope and scale to work the studen
MATTERS . ISSUE 18 – WINTER EDITION 2021. W elcome to the eighteenth issue of Analytical Matters, the e-newsletter of the Analytical Division of the Royal Society of Chemistry (RSC). Analytical Matters aims to showcase the wide range of analytical science activities being r
Association of Environmental Analytical Chemistry of India (AEACI ) was founded on 30th November, 2010 during a meeting held at Analytical Chemistry Division (ACD) in Bhabha Atomic Research Centre (BARC), Trombay, Mumbai to provide a common platform to all the Indian scientists and scholars working in the field of Analytical & Environmental .
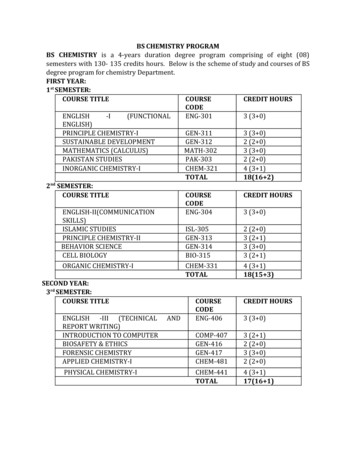
The Department of Chemistry, University of Turbat offers specialization in: I) Organic Chemistry II) Inorganic Chemistry III) Physical Chemistry IV) Biochemistry V) Analytical Chemistry 7 t h SEMESTER: INORGANIC CHEMISTRY COURSE TITLE COURSE CODE CREDIT HOURS PAPER-I (INORGANIC REACTION MECHANISM)
Analytical Chemistry Inorganic Chemistry 2nd edition Medicinal Chemistry Organic Chemistry 2nd edition Physical Chemistry . Section E— Chemistry in solution E1 Solvent types and properties 129 . The following Sections F–I cover different areas of the periodic table in a more descriptive way, although in .
Sep 05, 2014 · matter and energy, and carbon chemistry. Chemistry affects all aspects of life and most natural events because all living and nonliving things are made of matter. Five traditional areas of study are organic chemistry, inorganic chemistry, biochemistry, analytical chemistry, and physical chemistry
Chem 201 Analytical Chemistry- Prof. Dr. Şerife Yalçın Chemistry: The Central Science; all sub-disciplines rely on analytical chemistry to function. The interdisciplinary nature of chemical analysis makes it a vital tool in medical, industrial, government, and academic laboratories throughout the world.
Chemistry of Cycloalkanes 13. Chemistry of Alkyl halides 14. Alcohols 15. Chemistry of Ethers and Epoxides 16. Chemistry of Benzene and Aromaticity 17. Chemistry of Aryl Halides 18. Aromatic Sulphonic Acids 19. Chemistry of Aldehydes and Ketones 20. Carboxylic Acids 21. Chemistry of Carboxylic Acid Derivativ
chemistry unit 5 the mole answer key, chemistry matters unit 6d mole to mass calculations answers, unit 5 the mole and stoichiometry chemistry sv 0424-7 answers, chemistry unit 5 the mole answers, chemistry unit 8 worksheet 1 mole relationships answers, chemistry semester 2 review unit 9 the mole answers, chemistry
In Press Advanced Inorganic Chemistry Gurdeep Raj 06 270 Chromatography B.K. Sharma 06 Organic Chemistry 302 Advanced Organic Chemistry Aditi Singhal 08 279 Organic Chemistry Natural Products - Vol. I O. P. Agarwal 09 280 Organic Chemistry Natural Products - Vol. II O. P. Agarwal 09 281 Organic Chemistry Reactions & Reagents O. P. Agarwal 10
Chemistry Option A: Modern Analytical Chemistry Notes (HL & SL) 2014 Compiled from the IB Chemistry Guide for examinations beginning 2009, IB Past Papers, and Richard Thornley’s youtube channel f
B&W Radioisotope & Analytical Chemistry Laboratory, Lynchburg, VA B&W Y-12 Analytical Chemistry Organization, Oak Ridge, TN Radiological Materials Analysis Laboratory, ORNL UT-Battelle, LLC, Oak Ridge, TN Portsmouth Analytical Services, Piketon, OH Caltest Analytical Laboratory, Napa, CA
The main theme of Analytical Chemistry 2017 is “Showcasing the Contemporary Approaches in Analytical & Bioanalytical Techniques”. Analytical Chemistry 2017 stands for advertising of the products/services of the companies in the Composite Materials core sectors shall be
1. Basic theory of analytical chemistry (concentration, balancing, stoichiometry, acid-base, titration, oxidation-reduction, calibration, etc) will be reviewed. 2. Principles and applications of various analytical techniques used in modern analytical laboratory will be introduced. 3. These techniques include FTIR, UV/Vis, Raman, XPS,
Chemistry 103 and 104 provide a general background concerning the principles and factual basis of chemistry. The 103-104 sequence serves as a prerequisite for advanced courses such as Organic Chemistry (341 or 343), Analytical Chemistry (327 or 329), and Inorganic Chemistry (311). Students in Chemi
Trends in Care Delivery and Community Health State Public Health Leadership Webinar Deloitte Consulting LLP June 20, 2013. . Current state of Accountable Care Organizations (ACOs) and trends. Current state of Patient-Centered Medical Homes (PCMHs) and trends. Introduction.File Size: 2MBPage Count: 38Explore further2020 Healthcare Trends and How to Preparewww.healthcatalyst.comFive Health Care Trends For 2020 Health Affairswww.healthaffairs.orgTop 10 Emerging Trends in Health Care for 2021: The New .trustees.aha.orgRecommended to you b
Data Center Trends And Design. Data Center Trends & Design Agenda IT Trends Cooling Design Trends Power Design Trends. IT Trends Virtualization . increasing overall electrical efficiency by 2%. Reduces HVAC requirements by 6 tons/MW. Reduces the amount of equipment needed to support the load,
Course Name ANALYTICAL CHEMISTRY: ESSENTIAL METHODS Academic Unit SCHOOL OF CHEMISTRY . inquiry and analytical thinking abilities 3 Students are guided through several analytical techniques and instruments in the first half of the lab course (skills assessment). In the second half of the course, student have to combine techniques to solve a number of more complex problems (assessment by .
analytical chemistry in universities in the United States. Of significance is the increase in the number of PhDs, both in absolute terms and as a percentage of the total. In Northern Ireland there is a long established chair of analytical chemistry at Queen’s
Analytical Chemistry I is targeted at students pursuing higher education in the chemical sciences. The goal of this course is for students to master applying concepts and solving problems in analytical chemistry, with an emphasis on solution equilibria and electrochemistry.
TECHNIQUES IN ANALYTICAL CHEMISTRY FALL 2015 COURSE SYLLABUS . "Chemicals, Apparatus, and Unit Operation of Analytical Chemistry" in addition to the lab manual. . Redox in Biochemistry and the Environment. Make sure you can solve and understand examples***, 18-1, 18-2, 19-2 from the text book. .
BSc in Chemistry, Biological and Medicinal Chemistry (F152) Chemistry and Disease – Introduction to Medicinal Chemistry Proteins in 3D Chemistry and Disease – Advanced Medicinal Chemistry Genes and Genetic Engineering The following modules can then be used to make the number up to five: Bioinspired Chemistry, Proteins in Action, Synthesis – From Nature to the Lab. Departmental policies .
High School Chemistry is often a student’s first exposure to chemistry. You may not even be sure what “chemistry” really is. Many High Schools and Colleges are now requiring students to take High School Chemistry. This series will introduce you to the basic concepts and problem solving included every High School Chemistry Course, typically a two-semester class. Learning chemistry is .
JF Physical Chemistry 2013-2014. JF CH 1101: Introduction to Physical Chemistry . Professor Mike Lyons. School of Chemistry . Trinity College . Dublin 2. melyons@tcd.ie . A compendium of past examination questions set on Physical Chemistry on the JF Chemistry paper and problem sheets associated with CH1101 Physical Chemistry (Lyons) .
Textbook Essentials of Organic Chemistry by Dewick The following textbooks are also available in the chemistry library on reserve: Organic Chemistry: A Short Course by Hart, Craine, Hart and Hadid Introduction to Organic Chemistry by Brown and Poon Fundamentals of Organic Chemistry by McMurry Essential Organic Chemistry by Bru
From one of these Colorado public four-year institutions Adams State University [B.S. Chemistry] Colorado Mesa University [B.S. Chemistry] Colorado State University-Ft Collins [B.S. Chemistry] Colorado State University-Pueblo [B.S. Chemistry] Fort Lewis College [B.S. Chemistry; Chemistry option] Metropolitan State University of Denver
Keywords: analytical validation, pharmaceutical analysis, analytical method INTRODUCTION Analytical methods play an essential role in the adequate fulfillment of product quality attributes. However, the proper quality can only be reached if the analytical method undergoes an appropriate validation pr
Lifecycle Management of Analytical Methods Post-licensure activities for the method lifecycle management 1. Revalidation 2. Analytical Method Comparability Change in method (Method Replacement and modification) Analytical Method Transfer Post marketing changes to analytical pro
In-database analytical SQL with Oracle Database 12c This section outlines the high level processing concepts behind analytical SQL. Processing concepts behind analytical SQL Oracle’s in-database analytical SQL – first introduced in Oracle Database 8i Release



















![IND EX [krishnaprakashan ]](/img/170/chemistry-20content.jpg)


















