Chemistry Amp Treatment Of Cyanidation Wastes-PDF Free Download
PSI AP Physics 1 Name_ Multiple Choice 1. Two&sound&sources&S 1∧&S p;Hz&and250&Hz.&Whenwe& esult&is:& (A) great&&&&&(C)&The&same&&&&&
Argilla Almond&David Arrivederci&ragazzi Malle&L. Artemis&Fowl ColferD. Ascoltail&mio&cuore Pitzorno&B. ASSASSINATION Sgardoli&G. Auschwitzero&il&numero&220545 AveyD. di&mare Salgari&E. Avventurain&Egitto Pederiali&G. Avventure&di&storie AA.&VV. Baby&sitter&blues Murail&Marie]Aude Bambini&di&farina FineAnna
The program, which was designed to push sales of Goodyear Aquatred tires, was targeted at sales associates and managers at 900 company-owned stores and service centers, which were divided into two equal groups of nearly identical performance. For every 12 tires they sold, one group received cash rewards and the other received
College"Physics" Student"Solutions"Manual" Chapter"6" " 50" " 728 rev s 728 rpm 1 min 60 s 2 rad 1 rev 76.2 rad s 1 rev 2 rad , π ω π " 6.2 CENTRIPETAL ACCELERATION 18." Verify&that ntrifuge&is&about 0.50&km/s,∧&Earth&in&its& orbit is&about p;linear&speed&of&a .
Chemistry ORU CH 210 Organic Chemistry I CHE 211 1,3 Chemistry OSU-OKC CH 210 Organic Chemistry I CHEM 2055 1,3,5 Chemistry OU CH 210 Organic Chemistry I CHEM 3064 1 Chemistry RCC CH 210 Organic Chemistry I CHEM 2115 1,3,5 Chemistry RSC CH 210 Organic Chemistry I CHEM 2103 1,3 Chemistry RSC CH 210 Organic Chemistry I CHEM 2112 1,3
along with the gold values. Generally. the extraction of gold is accomplished by cyanidation in which leaching occurs by the addition of cyanide at alkaline pH. Cyanide, which is a strong lixiviant for gold, is an equally strong lixiviant for mercury. According to Sharpe (1), simple and complex cyanides of mercury are
theJazz&Band”∧&answer& musical&questions.&Click&on&Band .
Physical chemistry: Equilibria Physical chemistry: Reaction kinetics Inorganic chemistry: The Periodic Table: chemical periodicity Inorganic chemistry: Group 2 Inorganic chemistry: Group 17 Inorganic chemistry: An introduction to the chemistry of transition elements Inorganic chemistry: Nitrogen and sulfur Organic chemistry: Introductory topics
6" syl 4" syl 12" swgl @ 45 & 5' o.c. 12" swchl 6" swl r1-1 ma-d1-6a 4" syl 4" syl 2' 2' r3-5r r4-7 r&d 14.7' 13' cw open w11-15 w16-9p ma-d1-7d 12' 2' w4-3 moonwalks abb r&d r&d r&d r&d r&d r&d ret ret r&d r&d r&d r&d r&d 12' 24' r&d ma-d1-7a ma-d1-7b ret r&d r&d r5-1 r3-2 r&d r&r(b.o.) r6-1r r3-2 m4-5 m1-1 (i-195) m1-1 (i-495) m6-2l om1-1 .
s& . o Look at the poem’s first and last lines (first and last lines may give readers important . it is important to read poems four times. Remind them that the first time they read is for enjoyment; rereads allow them to dive deeper into poems .
Accelerated Chemistry I and Accelerated Chemistry Lab I and Accelerated Chemistry II and Accelerated Chemistry Lab II (preferred sequence) CHEM 102 & CHEM 103 & CHEM 104 & CHEM 105 General Chemistry I and General Chemistry Lab I and General Chemistry II and General Chemistry Lab II (with advisor approval) Organic chemistry, select from: 9-10
CHEM 0350 Organic Chemistry 1 CHEM 0360 Organic Chemistry 1 CHEM 0500 Inorganic Chemistry 1 CHEM 1140 Physical Chemistry: Quantum Chemistry 1 1 . Chemistry at Brown equivalent or greater in scope and scale to work the studen
Have&youheardabout&the& DCPublic&Library&Challenge?& Kids,teens,andadults&can have&funandwin ;by participating&inthe&2018&DC&Public .
Chemistry is the science that describes matter, its properties, the changes it undergoes, and the energy changes that accompany those processes. Inorganic chemistry Organic chemistry Physical chemistry Biochemistry Applied Chemistry: Analytical chemistry, Pharmaceutical Chemistry, . Istv an Szalai (E otv os University) Lecture 1 6 / 45
Chemistry of Cycloalkanes 13. Chemistry of Alkyl halides 14. Alcohols 15. Chemistry of Ethers and Epoxides 16. Chemistry of Benzene and Aromaticity 17. Chemistry of Aryl Halides 18. Aromatic Sulphonic Acids 19. Chemistry of Aldehydes and Ketones 20. Carboxylic Acids 21. Chemistry of Carboxylic Acid Derivativ
ADVANCED DIPLOMA Diploma in Chemistry 60% in Analytical Chemistry 3 Theory & Practical, Chemical Quality Assurance, Mathematics 2 Chemical Industrial 1 or S5 Subjects and Chemistry project II. Semester 1 Analytical Chemistry IV Physical Chemistry IV Research Methodology in Chemistry Semester 2 Inorganic Chemistry IV Organic Chemistry IV .
chemistry unit 5 the mole answer key, chemistry matters unit 6d mole to mass calculations answers, unit 5 the mole and stoichiometry chemistry sv 0424-7 answers, chemistry unit 5 the mole answers, chemistry unit 8 worksheet 1 mole relationships answers, chemistry semester 2 review unit 9 the mole answers, chemistry
Singapore*MOE*Math*Syllabus Wednesday, October 31, 12. w Grade 6 Concrete Pictorial . ms& and&numberrelationships&that&is& inning& insecondgrade andextendingallthe way&to&algebra& . Grade 6: ratio problems
Clients'!Self,Portraits! selves& & ce&(self:portraits),& letthem .
Creating A Webpage Using HTML & CSS ing&outcomes:& ;of&hypertext&markup&language& (HTML5)and .
A-R Guides After Reading lifying& previouslyheldbeliefs? Provide&reasoning& – Raonale&from&mulDple&sources& – Share∧&discuss& nave& orcompengperspecves 13 Completed A-R Sample 14
04 4 amp 05 5 amp 06 6 amp 07 7 amp 08 8 amp 10 10 amp 12 12 amp 13 13 amp 14 14 amp 15 15 amp 16 16 amp 20 20 amp line load20.887 [22.5] .016 [.4].887 [22.5] .016 [.4] Silver Printing On Black Embossed (ALUMINUM) 6 MOUNTING NUT 7 N None 1 Type 1 2 Type
behringer v-amp pro 19 2 hu 10 cm v-amp pro 8.2 v-amp pro 3 2 v-amp pro 180 ( 120 v) 9 9 8. 11 v-ampire/v-amp pro/v-amp 2 8 iec 8.1 xlr 8.2 6.3 mm 8.3 6.3 mm 8.4 8.3 midi midi 5 din v-amp pro midi midi midi inmidi edit midi out/thru midi out (midi thru) v-amp pro midi midi 8.3.1 midi-sysex v-amp pro edit midi (a ) midi sysex v-amp pro edit
The sequence of treatment processes through which wastewater passes is usually characterized as: 1. Preliminary treatment 2. Primary treatment 3. Secondary treatment 4. Tertiary treatment This discussion is an introduction to advanced treatment methods and processes. Advanced treatment is primarily a tertiary treatment.
The sequence of treatment processes through which wastewater passes is usually characterized as: 1. Preliminary treatment 2. Primary treatment 3. Secondary treatment 4. Tertiary treatment This discussion is an introduction to advanced treatment methods and processes. Advanced treatment is primarily a tertiary treatment.
BSc in Chemistry, Biological and Medicinal Chemistry (F152) Chemistry and Disease – Introduction to Medicinal Chemistry Proteins in 3D Chemistry and Disease – Advanced Medicinal Chemistry Genes and Genetic Engineering The following modules can then be used to make the number up to five: Bioinspired Chemistry, Proteins in Action, Synthesis – From Nature to the Lab. Departmental policies .
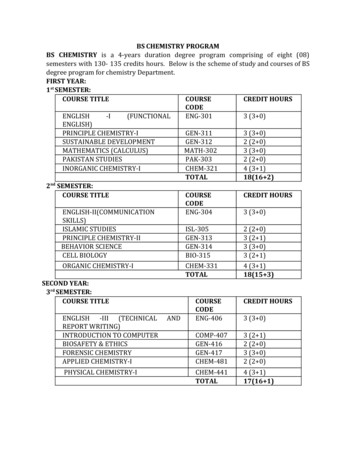
The Department of Chemistry, University of Turbat offers specialization in: I) Organic Chemistry II) Inorganic Chemistry III) Physical Chemistry IV) Biochemistry V) Analytical Chemistry 7 t h SEMESTER: INORGANIC CHEMISTRY COURSE TITLE COURSE CODE CREDIT HOURS PAPER-I (INORGANIC REACTION MECHANISM)
Analytical Chemistry Inorganic Chemistry 2nd edition Medicinal Chemistry Organic Chemistry 2nd edition Physical Chemistry . Section E— Chemistry in solution E1 Solvent types and properties 129 . The following Sections F–I cover different areas of the periodic table in a more descriptive way, although in .
High School Chemistry is often a student’s first exposure to chemistry. You may not even be sure what “chemistry” really is. Many High Schools and Colleges are now requiring students to take High School Chemistry. This series will introduce you to the basic concepts and problem solving included every High School Chemistry Course, typically a two-semester class. Learning chemistry is .
JF Physical Chemistry 2013-2014. JF CH 1101: Introduction to Physical Chemistry . Professor Mike Lyons. School of Chemistry . Trinity College . Dublin 2. melyons@tcd.ie . A compendium of past examination questions set on Physical Chemistry on the JF Chemistry paper and problem sheets associated with CH1101 Physical Chemistry (Lyons) .
Sep 05, 2014 · matter and energy, and carbon chemistry. Chemistry affects all aspects of life and most natural events because all living and nonliving things are made of matter. Five traditional areas of study are organic chemistry, inorganic chemistry, biochemistry, analytical chemistry, and physical chemistry
Textbook Essentials of Organic Chemistry by Dewick The following textbooks are also available in the chemistry library on reserve: Organic Chemistry: A Short Course by Hart, Craine, Hart and Hadid Introduction to Organic Chemistry by Brown and Poon Fundamentals of Organic Chemistry by McMurry Essential Organic Chemistry by Bru
From one of these Colorado public four-year institutions Adams State University [B.S. Chemistry] Colorado Mesa University [B.S. Chemistry] Colorado State University-Ft Collins [B.S. Chemistry] Colorado State University-Pueblo [B.S. Chemistry] Fort Lewis College [B.S. Chemistry; Chemistry option] Metropolitan State University of Denver
45 1068 opm sergio rieran&duran 22:45 g.a.lluisos&mataro 46 1235 vmb rafael valero&ortega 22:49 uca 47 1236 vmb manolo parejo&macho 23:04 uca 48 1054 vmb juan&pedro sanzcoca 23:08 g.a.lluisos&mataro 49 1037 opm jose&vicente reche&garcia 23:11 ca&vic 50 1360 vma luis molina&sanchez 23:14 jas 51 1161 opm efrem parera&coma 23:15 jab&berga
HAWG HOLSTERS MODEL LIST Don’t see your firearm? Just ask! We add new models every week. S&W Bodyguard 380 Crimson Trace Laser (green) S&W J-Frame S&W J-Frame .38 Special Snubnose Revolver S&W J-Frame Crimson Trace Laser Grip S&W Ladysmith 3913 S&W M&P 2.0 Compact S&W M&P 2.0 Compact 3.6" S&W M&P 2.0 Compact with LG362 Crimson Trace (red .
J - 70 Amp K - 80 Amp M - 90 Amp N - 100 Amp P - 125 Amp Q - 150 Amp R - 175 Amp S - 200 Amp T - 225 Amp U - 250 Amp V - 300 Amp
J - 70 Amp K - 80 Amp M - 90 Amp N - 100 Amp P - 125 Amp Q - 150 Amp R - 175 Amp S - 200 Amp T - 225 Amp U - 250 Amp V - 300 Amp
Linde V1400 MIG 400 Amp Miller Dimension 652 Amp Linde V160, 600 Amp Miller Dial Arc 250 Amp Linde V1800, 800 Amp Airco DCT 600 Amp Linde V1252, 205 Amp Airco Pulsed Arc 350 Amp Linde Heliarc UCC 305, 300 Amp Hobart MC 500 Amp Lincoln 3R3, 250 Amp Lincoln V350
4.1. Time of sowing by seed treatment 41 4.2. Cultivar by seed treatment 49 4.3. Time of harvest by seed treatment 57 4.4. Experimental treatment 60 5.0. Discussion 5.1. Time of sowing by seed treatment 64 5.2. Cultivar by seed treatment 68 5.3. Time of harvest by seed treatment 72 5.4. Expe
Tunisia Syria Armenia Albania Remote LMIC Country Study In-Depth LMIC Country Study In-Depth UMIC Country Study Remote UMIC Country Study . Country&case&studies& Interviews&with&5V10&key&informants& Vaccine&adopDon&decision&making& Regulatory&system& Financial&allocaDons& External&support